| Date | May 2018 | Marks available | 1 | Reference code | 18M.3.sl.TZ2.6 |
| Level | SL | Paper | 3 | Time zone | TZ2 |
| Command term | Outline | Question number | 6 | Adapted from | N/A |
Question
Lipids provide energy and are an important part of a balanced diet.
Identify the type of chemical reaction that occurs between fatty acids and glycerol to form lipids and the by-product of the reaction.
Arachidonic acid is a polyunsaturated omega-6 fatty acid found in peanut oil.
Determine the number of carbon–carbon double bonds present if the iodine number for the compound is 334. (Arachidonic acid Mr = 304.5)
Deduce the structure of the lipid formed by the reaction between lauric acid and glycerol (propane-1,2,3-triol) using section 34 of the data booklet.
Outline one impact food labelling has had on the consumption of foods containing different types of lipids.
Determine, to the correct number of significant figures, the energy produced by the respiration of 29.9 g of C5H10O5.
ΔHc (C5H10O5) = 205.9 kJ mol−1
Explain why lipids provide more energy than carbohydrates and proteins.
Markscheme
Type of reaction:
condensation
OR
esterification/triesterification
OR
nucleophilic substitution/nucleophilic displacement/SN2
By-product:
water/H2O
Do not accept just “substitution/displacement”.
[2 marks]
ALTERNATIVE 1
«\(\frac{{334}}{{253.8}}\) =» 1.32 AND «\(\frac{{100}}{{304.5}}\) =» 0.328
«\(\frac{{1.32}}{{0.328}}\) ≈» 4
ALTERNATIVE 2
«334 × \(\frac{{304.5}}{{100}}\) ≈» 1017
«\(\frac{{1017}}{{253.8}}\) ≈» 4
Award [2] for correct final answer.
[2 marks]
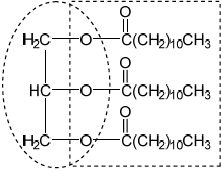
glycerol backbone
ester formula AND linkage
Accept a skeletal structure.
Penalize missing hydrogens or incorrect bond connectivities once only in Option B.
Accept condensed formula for ester.
[2 marks]
has affected consumption of trans-fats/cis-fats/saturated fats/unsaturated fats/hydrogenated/artificially altered fats
OR
reduce/eliminate trans-fats/increase in cis-fats
OR
reduce/eliminate saturated fats
OR
increase unsaturated fats
Do not accept “decrease in fat” alone.
Accept “lipid” for “fats”.
[1 mark]
«\(\frac{{29.9{\text{ g}}}}{{150.15{\text{ g mo}}{{\text{l}}^{ - 1}}}}\) =» 0.199 «mol»
«0.199 mol × 205.9 kJ mol–1 =» 41.0 «kJ»
Ignore significant figures in M1.
Award [2] for correct final answer.
Award [1 max] for incorrect significant figures in final answer.
[2 marks]
ratio of oxygen to carbon in lipids lower
OR
lipids less oxidized
OR
lipids more reduced
more energy per mass/g released when lipids are oxidized
Accept “«average» oxidation number of carbon in linoleic acid is lower” for M1.
[2 marks]

