| Date | May 2011 | Marks available | 2 | Reference code | 11M.3.sl.TZ1.B2 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Explain | Question number | B2 | Adapted from | N/A |
Question
Determine the number of double bonds in linoleic acid, \({{\text{C}}_{{\text{18}}}}{{\text{H}}_{{\text{32}}}}{{\text{O}}_{\text{2}}}\), and linolenic acid, \({{\text{C}}_{{\text{18}}}}{{\text{H}}_{{\text{30}}}}{{\text{O}}_{\text{2}}}\), and suggest which fatty acid will have a higher iodine number.
Explain why it is important to include the fatty acids linoleic and linolenic acid in a balanced diet.
The partial equation for the enzyme-catalysed hydrolysis of a triglyceride is shown below. Draw the structural formulas of the products A and B.
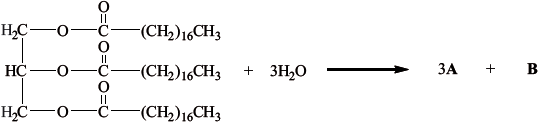
A:
B:
Deduce whether the fatty acid obtained in part (c) will have a higher or lower melting point compared to oleic acid, \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{\text{7}}}{\text{CH=CH(C}}{{\text{H}}_{\text{2}}}{{\text{)}}_{\text{7}}}{\text{COOH}}\). Outline your reason.
Markscheme
linoleic has 2 C=C / double bonds and linolenic has 3 C=C / double bonds;
linolenic acid (will have higher iodine value);
Accept linoleic has 3 double bonds and linolenic has 4 double bonds.
essential fatty acids / cannot be synthesized in body;
lowers LDL cholesterol level / lowers risk of heart disease / affects inflammation / conversion to important molecules;
A: \({\text{C}}{{\text{H}}_{\text{3}}}{{\text{(C}}{{\text{H}}_{\text{2}}}{\text{)}}_{{\text{16}}}}{\text{COOH}}\);
B: \({\text{C}}{{\text{H}}_{\text{2}}}{\text{OHCHOHC}}{{\text{H}}_{\text{2}}}{\text{OH}}\);
Accept [1 max] if A and B reversed.
Accept full structural formula.
Penalize missing H atoms once only.
higher (melting point);
saturated fatty acids / no unsaturation / no C=C bonds;
Accept appropriate reason such as close packing, no kink in molecule, stronger van der Waals’ forces, larger surface area of contact.
Accept opposite reasons why oleic acid would have a lower mp.
Examiners report
Many candidates correctly identified the number of double bonds present from the molecular formula and could link this to the iodine number, but fewer knew that these were essential fatty acids (that is ones we cannot synthesise) and the way in which they are used in the body. It was surprising how few students could correctly identify the hydrolysis products of a triglyceride and, though many were aware of the links between structure and melting point, explaining this concisely sometimes proved to be a challenge.
Many candidates correctly identified the number of double bonds present from the molecular formula and could link this to the iodine number, but fewer knew that these were essential fatty acids (that is ones we cannot synthesise) and the way in which they are used in the body. It was surprising how few students could correctly identify the hydrolysis products of a triglyceride and, though many were aware of the links between structure and melting point, explaining this concisely sometimes proved to be a challenge.
Many candidates correctly identified the number of double bonds present from the molecular formula and could link this to the iodine number, but fewer knew that these were essential fatty acids (that is ones we cannot synthesise) and the way in which they are used in the body. It was surprising how few students could correctly identify the hydrolysis products of a triglyceride and, though many were aware of the links between structure and melting point, explaining this concisely sometimes proved to be a challenge.
Many candidates correctly identified the number of double bonds present from the molecular formula and could link this to the iodine number, but fewer knew that these were essential fatty acids (that is ones we cannot synthesise) and the way in which they are used in the body. It was surprising how few students could correctly identify the hydrolysis products of a triglyceride and, though many were aware of the links between structure and melting point, explaining this concisely sometimes proved to be a challenge.

