| Date | May 2013 | Marks available | 2 | Reference code | 13M.3.sl.TZ2.B1 |
| Level | SL | Paper | 3 | Time zone | TZ2 |
| Command term | Explain | Question number | B1 | Adapted from | N/A |
Question
Lipids play a significant role in human nutrition and have many important biological functions. The triglycerides are one type of lipid.
Table 22 of the Data Booklet shows the formulas of some fatty acids.
Olive oil contains a triglyceride (glyceryl trioleate) which, on hydrolysis, yields propane-1,2,3-triol (glycerol) and oleic acid.
Deduce the equation for this reaction. You may use the letter R to represent the hydrocarbon chains.
Calculate the iodine number for oleic acid (\({M_{\text{r}}}\) of oleic acid \( = 282.52\)).
Linoleic acid and stearic acid have similar molecular masses. Explain why linoleic acid has a much lower melting point than stearic acid.
Linoleic acid and linolenic acid are classed as essential fatty acids. State the importance of these fatty acids in the human diet.
Markscheme
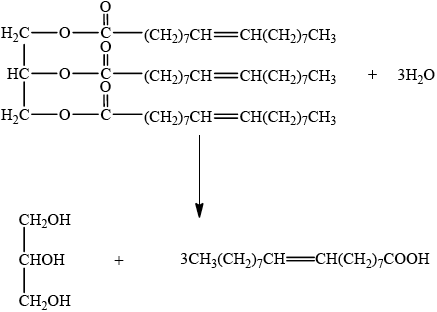
correct structure for triglyceride;
correct structures for products;
correct balancing;
Mark for balancing can only be awarded if reactants and products are correct.
Accept more condensed structural formula, but ester group must be the correct
way round (glycerol–OOC–R or glycerol–O–CO–R, not glycerol–COO–R).
Do not penalize minor errors in the hydrocarbon chain or in the use of R.
100 g of oleic acid reacts with \(\frac{{{\text{253.8}} \times {\text{100}}}}{{{\text{282.52}}}}{\text{ (g)}}\) of \({{\text{I}}_{\text{2}}}\);
Do not penalize use of integer values for \({M_r}\).
hence iodine number is 89.9;
Accept answers between 89.7 and 90.
Award [2] for for correct answer.
Award [1] for an iodine number between 44.8 and 45.0 if monatomic iodine is assumed.
Award [1] for an iodine number between 0.897 and 0.900 if 1 g of oleic acid assumed.
Award [1] for an iodine number between 111 and 112 – mass of I2 reacting with 100 g of oleic acid.
in linoleic acid, presence of C=C/double bond/unsaturation prevents close packing/leads to kinks/bends in chain;
Do not allow mark without reference to C=C/double bond/unsaturation.
hence weaker van der Waals’/London/dispersion forces between molecules;
Accept opposite statements for stearic acid but must point out that this is because it does not have a C=C/double bond/unsaturation.
cannot be synthesized by body;
Accept specific uses such as lowers LDL cholesterol level, increases HDL
cholesterol level or lowers risk of heart disease.
Do not accept just “lowers cholesterol level”.
Examiners report
This proved surprisingly challenging. Even using R for the hydrocarbon chain, many candidates found drawing the structure of a triglyceride a challenge and only a handful correctly balanced the equation by adding an appropriate number of water molecules. From the way in which the calculation was tackled, very few knew the definition of iodine number and there were even less correct answers. Few students gained marks for the early steps of the calculation because their working was rarely clear. The effect of the double bond on packing was better known, as was the importance of essential fatty acids.
This proved surprisingly challenging. Even using R for the hydrocarbon chain, many candidates found drawing the structure of a triglyceride a challenge and only a handful correctly balanced the equation by adding an appropriate number of water molecules. From the way in which the calculation was tackled, very few knew the definition of iodine number and there were even less correct answers. Few students gained marks for the early steps of the calculation because their working was rarely clear. The effect of the double bond on packing was better known, as was the importance of essential fatty acids.
This proved surprisingly challenging. Even using R for the hydrocarbon chain, many candidates found drawing the structure of a triglyceride a challenge and only a handful correctly balanced the equation by adding an appropriate number of water molecules. From the way in which the calculation was tackled, very few knew the definition of iodine number and there were even less correct answers. Few students gained marks for the early steps of the calculation because their working was rarely clear. The effect of the double bond on packing was better known, as was the importance of essential fatty acids.
This proved surprisingly challenging. Even using R for the hydrocarbon chain, many candidates found drawing the structure of a triglyceride a challenge and only a handful correctly balanced the equation by adding an appropriate number of water molecules. From the way in which the calculation was tackled, very few knew the definition of iodine number and there were even less correct answers. Few students gained marks for the early steps of the calculation because their working was rarely clear. The effect of the double bond on packing was better known, as was the importance of essential fatty acids.

