| Date | November 2014 | Marks available | 3 | Reference code | 14N.3.sl.TZ0.22 |
| Level | SL | Paper | 3 | Time zone | TZ0 |
| Command term | Comment and State | Question number | 22 | Adapted from | N/A |
Question
Most foods contain nutrients.
Triglycerides are formed by the reaction of propane-1,2,3-triol (glycerol) with fatty acids.
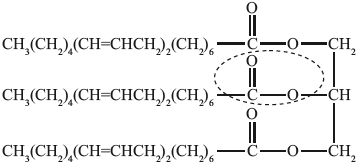
(i) State the name of the functional group circled in the triglyceride.
(ii) Identify the other product of the reaction.
(i) State the difference in structure between the fatty acids found in an oil and those in a fat.
(ii) Comment on the relative stability of oils and fats and state the names of two possible types of degradation reaction.
Markscheme
(i) ester;
(ii) water/\({{\text{H}}_{\text{2}}}{\text{O}}\);
(i) (fatty acids in) oils are unsaturated/contain (many) C=C/carbon-carbon double bonds / (fatty acids in) fats are (mostly) saturated/contain no/few/fewer (than oils) C=C /carbon-carbon double bonds;
(ii) C=C bonds degrade/oxidize more rapidly / oils become rancid more rapidly / fats are more stable;
Award [1 max] for any two of:
auto-oxidation;
Allow oxidative rancidity.
Do not accept “reaction with oxygen” (name required).
photo-oxidation;
Do not accept light.
microbial rancidity;
hydrolysis;
Allow hydrolytic rancidity.
Do not accept “addition of water” (name required).
Do not accept hydrogenation (since not a degradation reaction).
Examiners report
In (b) (i), the better candidates stated ester. The weaker candidates incorrectly suggested either alcohol or carboxylic acid. Water was universally known in (ii).
(c) was well answered though some did not score full marks by suggesting that hydrogenation is a degradation reaction which is incorrect.

