| Date | May 2010 | Marks available | 2 | Reference code | 10M.3.hl.TZ2.B4 |
| Level | HL | Paper | 3 | Time zone | TZ2 |
| Command term | Calculate | Question number | B4 | Adapted from | N/A |
Question
Calculate the number of carbon-carbon double bonds in linolenic acid, \({{\text{C}}_{{\text{18}}}}{{\text{H}}_{{\text{30}}}}{{\text{O}}_{\text{2}}}\), given that 7.7 g of iodine, \({{\text{I}}_{\text{2}}}\), react with 2.8 g of linolenic acid.
Markscheme
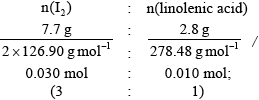
3 C=C double bonds;
3 C=C double bonds scores [2].
No ECF.
Examiners report
Many candidates were able to solve this straight forward calculation question. The major errors candidates made included comparing the ratio of the masses provided rather than working out the ratio of the number of moles of linolenic acid to iodine. In this instance candidates were not awarded any marks because they clearly did not understand the concepts involved. Candidates could also calculate the number of carbon-carbon bonds from the formula of linolenic acid.
Syllabus sections
- 17N.3.sl.TZ0.8c: Outline the importance of linoleic acid for human health.
- 17N.3.sl.TZ0.8b.ii: Calculate the volume of iodine solution used to reach the end-point.
- 17N.3.sl.TZ0.8b.i: State the type of reaction occurring during the titration.
- 17N.3.sl.TZ0.8a.ii: The empirical formula of fructose is CH2O. Suggest why linoleic acid releases more energy per...
- 17M.3.sl.TZ2.9b: Identify the type of reaction which occurs.
- 17M.3.sl.TZ2.9a: Glycerol is one product of the reaction. Identify the two other organic products.
- 17M.3.sl.TZ2.8b: 10.0 g of sunflower oil reacts completely with 123 cm3 of 0.500 mol\(\,\)dm–3 iodine...
- 17M.3.sl.TZ2.8a: Explain which one of these fatty acids has the highest boiling point.
- 17M.3.sl.TZ1.11c: The amount of proteins, fats and carbohydrates determine the energy content of...
- 17M.3.sl.TZ1.11b.ii: Solid fat triglycerides can also clog kitchen sink drains. Explain how sodium hydroxide...
- 17M.3.sl.TZ1.11b.i: The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid,...
- 17M.3.sl.TZ1.11a: List the building blocks of triglycerides and carbohydrates.
- 16N.3.sl.TZ0.8b: The table below shows average figures for the percentage fatty acid composition of some...
- 16N.3.sl.TZ0.8a: Fatty acids react with glycerol to form fats and oils. State the name of the chemical link...
- 16M.3.sl.TZ0.8c: The production of banned steroids has ethical implications. Suggest a reason why steroid...
- 16M.3.sl.TZ0.8b: (i) State the name of the functional group circled in the DHEA molecule shown below. (ii)...
- 16M.3.sl.TZ0.8a: Steroid abuse has certain health hazards, some general, some specific to males and some...
- 15M.3.hl.TZ2.8b: Calculate the iodine number for linolenic acid,...
- 15M.3.hl.TZ2.26b.ii: Discuss two effects on health of consuming trans fatty acids such as elaidic acid.
- 15M.3.sl.TZ1.6a: A number of famous athletes have been banned from competition for using hormone F. Explain,...
- 15M.3.sl.TZ1.5a: Compare the structures and polarities of fats and phospholipids, giving one similarity and...
- 15M.3.sl.TZ1.5b.i: Vitamin D is produced from cholesterol. The structures of both molecules are given in table...
- 15M.3.sl.TZ1.5b.ii: Distinguish between HDL and LDL cholesterol in terms of their composition and their effect on...
- 15M.3.sl.TZ1.17b.i: Describe one similarity and one difference between the structure of a saturated and an...
- 15M.3.sl.TZ2.7a: List two benefits of linolenic acid to humans.
- 15M.3.sl.TZ2.7b.ii: Calculate the iodine number for linolenic acid, C17H29COOH \(({M_{\text{r}}} =...
- 15M.3.sl.TZ2.9a: Aldosterone is one of the steroid hormones produced in the body from cholesterol. The...
- 15M.3.sl.TZ2.22a: State the name of the compound which combines with fatty acids to form triglycerides.
- 15M.3.sl.TZ2.22b.ii: Discuss two effects on health of consuming trans fatty acids such as elaidic acid.
- 15M.3.sl.TZ1.18b: Explain, giving their names, the two types of reaction by which foods may become...
- 14M.3.sl.TZ1.18a: Identify the structural formula of the triglyceride formed when three molecules of linoleic...
- 14M.3.sl.TZ1.18b: State the other product formed during this reaction.
- 14M.3.sl.TZ1.18c: Explain why the triglyceride formed from linoleic acid and glycerol is a liquid and not a...
- 14M.3.sl.TZ2.5a: (i) Define the term iodine number. (ii) A sample containing...
- 14M.3.sl.TZ2.5d: Explain why the metabolism of fats produces much more energy per gram than that of...
- 14M.3.sl.TZ2.5c: The hydrolysis of tristearin, whose structure is shown below, can be catalysed by the enzyme...
- 14M.3.sl.TZ2.17b: State two named functional groups present in each of the following molecules found in two...
- 14M.3.sl.TZ2.17c: Butter is an example of a saturated fat and olive oil is an example of an unsaturated fat....
- 14N.3.hl.TZ0.5: Compare the structures and chemical formulas of the two essential fatty acids linoleic acid...
- 14N.3.sl.TZ0.22c: (i) State the difference in structure between the fatty acids found in an oil and those...
- 14N.3.sl.TZ0.4a: Define the term iodine number.
- 14N.3.sl.TZ0.4b: Diets that are high in omega-3 fatty acids are recommended as healthy for the heart....
- 14N.3.sl.TZ0.22b: (i) State the name of the functional group circled in the triglyceride. (ii) ...
- 13N.3.hl.TZ0.8b: State the other reactant and one essential condition that would favour this hydrolysis...
- 13N.3.hl.TZ0.8c: Identify which product is polyunsaturated, and outline why foods containing this type of...
- 13N.3.hl.TZ0.8a: Draw a possible structure for the triglyceride.
- 13N.3.hl.TZ0.24a: Explain which acid has the highest melting point.
- 13N.3.sl.TZ0.7a: Draw a possible structure for the triglyceride.
- 13N.3.sl.TZ0.7b: State the other reactant and one essential condition that would favour this hydrolysis...
- 13N.3.sl.TZ0.7c: Identify which product is polyunsaturated, and outline why foods containing this type of...
- 13N.3.sl.TZ0.7d: People who live in very cold regions need a diet with a higher ratio of fat to carbohydrate...
- 13N.3.sl.TZ0.20a: Explain which acid has the highest melting point.
- 13M.3.sl.TZ1.F1c: Describe the rancidity of fats.
- 13M.3.sl.TZ2.B1b.i: Linoleic acid and stearic acid have similar molecular masses. Explain why linoleic acid has a...
- 13M.3.sl.TZ2.B1b.ii: Linoleic acid and linolenic acid are classed as essential fatty acids. State the importance...
- 13M.3.sl.TZ2.B1a.i: Olive oil contains a triglyceride (glyceryl trioleate) which, on hydrolysis, yields...
- 13M.3.sl.TZ2.B1a.ii: Calculate the iodine number for oleic acid (\({M_{\text{r}}}\) of oleic acid \( = 282.52\)).
- 12N.3.sl.TZ0.B2b: Describe, by completing the equation below, the condensation of glycerol and the three fatty...
- 12N.3.sl.TZ0.B2c: (i) State the names of two other types of lipids present in the human body. (ii) ...
- 12N.3.sl.TZ0.F1a: (i) all fats. (ii) all fatty acids.
- 10N.3.sl.TZ0.B2a: Linoleic acid is an essential fatty acid whose formula is given in Table 22 of the Data...
- 10N.3.sl.TZ0.B2b: Fats, such as butter, are solid triglycerides. Explain why fats have a higher energy value...
- 09N.3.sl.TZ0.F1c: Liver is a source of arachidonic acid,...
- 09N.3.sl.TZ0.F1b.i: fats and oils
- 10M.3.sl.TZ1.B3: (a) Define the term iodine number. (b) Linoleic acid (\({M_{\text{r}}} = 281\)) has...
- 10M.3.sl.TZ1.F3a: Give the general structural formula for a fat or oil and describe the difference in structure...
- 10M.3.sl.TZ1.F3c: Oils can be hydrogenated. One possible problem is that partial hydrogenation may occur which...
- 10M.3.sl.TZ1.F3b: Explain why unsaturated fats have a lower melting point than saturated fats.
- 10M.3.sl.TZ2.B4: (a) Outline the function and production of hormones in the body. (b) In many...
- 10M.3.sl.TZ2.F2: (a) Describe the differences in the structure between the saturated fatty acid...
- 09M.3.hl.TZ1.B2b.ii: Calculate the iodine number of linoleic...
- 09M.3.sl.TZ1.B2b.i: Compare the structures of the two fatty acids: linoleic and linolenic acids.
- 09M.3.sl.TZ1.B2b.ii: State why these two fatty acids are so important in the human diet.
- 09M.3.sl.TZ1.B2a: The formulas of some fatty acids are shown in Table 22 of the Data Booklet. State the...
- 09M.3.sl.TZ1.B2c.i: Distinguish between HDL and LDL cholesterol.
- 09M.3.sl.TZ1.B2c.ii: Compare the composition of cholesterol with a phospholipid such as lecithin.
- 09M.3.hl.TZ2.B2d: Describe one negative effect of a high concentration of LDL cholesterol in blood.
- 09M.3.sl.TZ2.B2b: Identify two other types of lipids found in the human body.
- 09M.3.sl.TZ2.B2c.i: State what the terms HDL and LDL represent.
- 09M.3.sl.TZ2.B2d: Compare the structures of linoleic acid and linolenic acid.
- 09M.3.sl.TZ2.F1a: Describe the chemical composition of a triglyceride.
- 09M.3.sl.TZ2.B2a: Identify the characteristic structural feature of cholesterol.
- 09M.3.sl.TZ2.F1b: The following two structures represent isomers of a fatty acid. State and explain which...
- 11M.3.sl.TZ1.B2c: The partial equation for the enzyme-catalysed hydrolysis of a triglyceride is shown below....
- 11M.3.sl.TZ1.F1a: Deduce which fat or oil from the table could best be described...
- 11M.3.sl.TZ1.B2a: Determine the number of double bonds in linoleic acid,...
- 11M.3.sl.TZ1.B2b: Explain why it is important to include the fatty acids linoleic and linolenic acid in a...
- 11M.3.sl.TZ1.B2d: Deduce whether the fatty acid obtained in part (c) will have a higher or lower melting point...
- 11M.3.sl.TZ2.B1e: Explain why fats have a higher energy value per mole than carbohydrates.
- 11M.3.sl.TZ2.F1a.i: Predict the products of hydrolytic rancidity of fats.
- 11M.3.sl.TZ2.B1c: Explain whether the triglyceride in part (b) is a solid or a liquid at room temperature.
- 11M.3.sl.TZ2.B1d: Identify the type of reaction that occurs during the formation of a triglyceride.
- 11M.3.sl.TZ2.B1a: Identify the compounds X and Y. X: Y:
- 11M.3.sl.TZ2.B1b: Draw the structural formula of a triglyceride formed from one molecule each of octanoic acid,...
- 11M.3.sl.TZ2.F1a.ii: The hydrolysis of milk products is used in the making of cheese. State two conditions which...
- 12M.3.sl.TZ1.B1b: This energy comes mainly from the combustion of triglycerides. State the name of one other...
- 12M.3.sl.TZ1.F1b: Identify the types of nutrients A, B and C. A B C
- 12M.3.sl.TZ1.B1c: Explain why lipids have a higher energy content than carbohydrates.
- 12M.3.sl.TZ1.F2a: Rancidity can occur as a result of two separate processes. State these processes and explain...
- 12M.3.sl.TZ2.B3a: (i) Identify the major source of low-density lipoproteins. (ii) State the importance...
- 12M.3.sl.TZ2.B3b.i: Compare the chemical structures of linoleic acid, an omega-6 fatty acid, and linolenic acid,...
- 12M.3.sl.TZ2.F2a: State two major differences in their structures.
- 12M.3.sl.TZ2.F2c: Discuss two advantages and two disadvantages of converting oils into fats.
- 11N.3.sl.TZ0.F1b.ii: Deduce the structural formula of a triester formed from three long-chain carboxylic acid...
- 11N.3.sl.TZ0.F2a: State the meaning of the term rancidity as it applies to fats.
- 11N.3.sl.TZ0.F2b.i: Compare the two rancidity processes. Hydrolytic process: Oxidative process:

