| Date | May 2015 | Marks available | 2 | Reference code | 15M.3.sl.TZ1.6 |
| Level | SL | Paper | 3 | Time zone | TZ1 |
| Command term | Explain | Question number | 6 | Adapted from | N/A |
Question
F and G are two synthetic hormones. The structures of some natural hormones are given in table 21 of the data booklet.
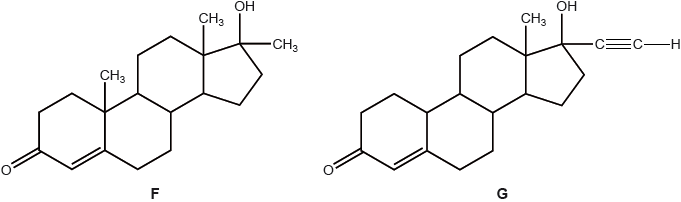
A number of famous athletes have been banned from competition for using hormone F.
Explain, with reference to its structure, why hormone F improves performance.
Markscheme
structure/function similar to testosterone;
causes increased rate of protein synthesis/tissue/muscle building/increase in muscle mass / OWTTE;
Accept “anabolic” for M2.
Examiners report
Generally well-answered. Some candidates thought hormone F was testosterone.
Syllabus sections
Show 106 related questions
- 17N.3.sl.TZ0.8c: Outline the importance of linoleic acid for human health.
- 17N.3.sl.TZ0.8b.ii: Calculate the volume of iodine solution used to reach the end-point.
- 17N.3.sl.TZ0.8b.i: State the type of reaction occurring during the titration.
- 17N.3.sl.TZ0.8a.ii: The empirical formula of fructose is CH2O. Suggest why linoleic acid releases more energy per...
- 17M.3.sl.TZ2.9b: Identify the type of reaction which occurs.
- 17M.3.sl.TZ2.9a: Glycerol is one product of the reaction. Identify the two other organic products.
- 17M.3.sl.TZ2.8b: 10.0 g of sunflower oil reacts completely with 123 cm3 of 0.500 mol\(\,\)dm–3 iodine...
- 17M.3.sl.TZ2.8a: Explain which one of these fatty acids has the highest boiling point.
- 17M.3.sl.TZ1.11c: The amount of proteins, fats and carbohydrates determine the energy content of...
- 17M.3.sl.TZ1.11b.ii: Solid fat triglycerides can also clog kitchen sink drains. Explain how sodium hydroxide...
- 17M.3.sl.TZ1.11b.i: The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid,...
- 17M.3.sl.TZ1.11a: List the building blocks of triglycerides and carbohydrates.
- 16N.3.sl.TZ0.8b: The table below shows average figures for the percentage fatty acid composition of some...
- 16N.3.sl.TZ0.8a: Fatty acids react with glycerol to form fats and oils. State the name of the chemical link...
- 16M.3.sl.TZ0.8c: The production of banned steroids has ethical implications. Suggest a reason why steroid...
- 16M.3.sl.TZ0.8b: (i) State the name of the functional group circled in the DHEA molecule shown below. (ii)...
- 16M.3.sl.TZ0.8a: Steroid abuse has certain health hazards, some general, some specific to males and some...
- 15M.3.hl.TZ2.8b: Calculate the iodine number for linolenic acid,...
- 15M.3.hl.TZ2.26b.ii: Discuss two effects on health of consuming trans fatty acids such as elaidic acid.
- 15M.3.sl.TZ1.5a: Compare the structures and polarities of fats and phospholipids, giving one similarity and...
- 15M.3.sl.TZ1.5b.i: Vitamin D is produced from cholesterol. The structures of both molecules are given in table...
- 15M.3.sl.TZ1.5b.ii: Distinguish between HDL and LDL cholesterol in terms of their composition and their effect on...
- 15M.3.sl.TZ1.17b.i: Describe one similarity and one difference between the structure of a saturated and an...
- 15M.3.sl.TZ2.7a: List two benefits of linolenic acid to humans.
- 15M.3.sl.TZ2.7b.ii: Calculate the iodine number for linolenic acid, C17H29COOH \(({M_{\text{r}}} =...
- 15M.3.sl.TZ2.9a: Aldosterone is one of the steroid hormones produced in the body from cholesterol. The...
- 15M.3.sl.TZ2.22a: State the name of the compound which combines with fatty acids to form triglycerides.
- 15M.3.sl.TZ2.22b.ii: Discuss two effects on health of consuming trans fatty acids such as elaidic acid.
- 15M.3.sl.TZ1.18b: Explain, giving their names, the two types of reaction by which foods may become...
- 14M.3.sl.TZ1.18a: Identify the structural formula of the triglyceride formed when three molecules of linoleic...
- 14M.3.sl.TZ1.18b: State the other product formed during this reaction.
- 14M.3.sl.TZ1.18c: Explain why the triglyceride formed from linoleic acid and glycerol is a liquid and not a...
- 14M.3.sl.TZ2.5a: (i) Define the term iodine number. (ii) A sample containing...
- 14M.3.sl.TZ2.5d: Explain why the metabolism of fats produces much more energy per gram than that of...
- 14M.3.sl.TZ2.5c: The hydrolysis of tristearin, whose structure is shown below, can be catalysed by the enzyme...
- 14M.3.sl.TZ2.17b: State two named functional groups present in each of the following molecules found in two...
- 14M.3.sl.TZ2.17c: Butter is an example of a saturated fat and olive oil is an example of an unsaturated fat....
- 14N.3.hl.TZ0.5: Compare the structures and chemical formulas of the two essential fatty acids linoleic acid...
- 14N.3.sl.TZ0.22c: (i) State the difference in structure between the fatty acids found in an oil and those...
- 14N.3.sl.TZ0.4a: Define the term iodine number.
- 14N.3.sl.TZ0.4b: Diets that are high in omega-3 fatty acids are recommended as healthy for the heart....
- 14N.3.sl.TZ0.22b: (i) State the name of the functional group circled in the triglyceride. (ii) ...
- 13N.3.hl.TZ0.8b: State the other reactant and one essential condition that would favour this hydrolysis...
- 13N.3.hl.TZ0.8c: Identify which product is polyunsaturated, and outline why foods containing this type of...
- 13N.3.hl.TZ0.8a: Draw a possible structure for the triglyceride.
- 13N.3.hl.TZ0.24a: Explain which acid has the highest melting point.
- 13N.3.sl.TZ0.7a: Draw a possible structure for the triglyceride.
- 13N.3.sl.TZ0.7b: State the other reactant and one essential condition that would favour this hydrolysis...
- 13N.3.sl.TZ0.7c: Identify which product is polyunsaturated, and outline why foods containing this type of...
- 13N.3.sl.TZ0.7d: People who live in very cold regions need a diet with a higher ratio of fat to carbohydrate...
- 13N.3.sl.TZ0.20a: Explain which acid has the highest melting point.
- 13M.3.sl.TZ1.F1c: Describe the rancidity of fats.
- 13M.3.sl.TZ2.B1b.i: Linoleic acid and stearic acid have similar molecular masses. Explain why linoleic acid has a...
- 13M.3.sl.TZ2.B1b.ii: Linoleic acid and linolenic acid are classed as essential fatty acids. State the importance...
- 13M.3.sl.TZ2.B1a.i: Olive oil contains a triglyceride (glyceryl trioleate) which, on hydrolysis, yields...
- 13M.3.sl.TZ2.B1a.ii: Calculate the iodine number for oleic acid (\({M_{\text{r}}}\) of oleic acid \( = 282.52\)).
- 12N.3.sl.TZ0.B2b: Describe, by completing the equation below, the condensation of glycerol and the three fatty...
- 12N.3.sl.TZ0.B2c: (i) State the names of two other types of lipids present in the human body. (ii) ...
- 12N.3.sl.TZ0.F1a: (i) all fats. (ii) all fatty acids.
- 10N.3.sl.TZ0.B2a: Linoleic acid is an essential fatty acid whose formula is given in Table 22 of the Data...
- 10N.3.sl.TZ0.B2b: Fats, such as butter, are solid triglycerides. Explain why fats have a higher energy value...
- 09N.3.sl.TZ0.F1c: Liver is a source of arachidonic acid,...
- 09N.3.sl.TZ0.F1b.i: fats and oils
- 10M.3.sl.TZ1.B3: (a) Define the term iodine number. (b) Linoleic acid (\({M_{\text{r}}} = 281\)) has...
- 10M.3.sl.TZ1.F3a: Give the general structural formula for a fat or oil and describe the difference in structure...
- 10M.3.sl.TZ1.F3c: Oils can be hydrogenated. One possible problem is that partial hydrogenation may occur which...
- 10M.3.sl.TZ1.F3b: Explain why unsaturated fats have a lower melting point than saturated fats.
- 10M.3.hl.TZ2.B4: Calculate the number of carbon-carbon double bonds in linolenic acid,...
- 10M.3.sl.TZ2.B4: (a) Outline the function and production of hormones in the body. (b) In many...
- 10M.3.sl.TZ2.F2: (a) Describe the differences in the structure between the saturated fatty acid...
- 09M.3.hl.TZ1.B2b.ii: Calculate the iodine number of linoleic...
- 09M.3.sl.TZ1.B2b.i: Compare the structures of the two fatty acids: linoleic and linolenic acids.
- 09M.3.sl.TZ1.B2b.ii: State why these two fatty acids are so important in the human diet.
- 09M.3.sl.TZ1.B2a: The formulas of some fatty acids are shown in Table 22 of the Data Booklet. State the...
- 09M.3.sl.TZ1.B2c.i: Distinguish between HDL and LDL cholesterol.
- 09M.3.sl.TZ1.B2c.ii: Compare the composition of cholesterol with a phospholipid such as lecithin.
- 09M.3.hl.TZ2.B2d: Describe one negative effect of a high concentration of LDL cholesterol in blood.
- 09M.3.sl.TZ2.B2b: Identify two other types of lipids found in the human body.
- 09M.3.sl.TZ2.B2c.i: State what the terms HDL and LDL represent.
- 09M.3.sl.TZ2.B2d: Compare the structures of linoleic acid and linolenic acid.
- 09M.3.sl.TZ2.F1a: Describe the chemical composition of a triglyceride.
- 09M.3.sl.TZ2.B2a: Identify the characteristic structural feature of cholesterol.
- 09M.3.sl.TZ2.F1b: The following two structures represent isomers of a fatty acid. State and explain which...
- 11M.3.sl.TZ1.B2c: The partial equation for the enzyme-catalysed hydrolysis of a triglyceride is shown below....
- 11M.3.sl.TZ1.F1a: Deduce which fat or oil from the table could best be described...
- 11M.3.sl.TZ1.B2a: Determine the number of double bonds in linoleic acid,...
- 11M.3.sl.TZ1.B2b: Explain why it is important to include the fatty acids linoleic and linolenic acid in a...
- 11M.3.sl.TZ1.B2d: Deduce whether the fatty acid obtained in part (c) will have a higher or lower melting point...
- 11M.3.sl.TZ2.B1e: Explain why fats have a higher energy value per mole than carbohydrates.
- 11M.3.sl.TZ2.F1a.i: Predict the products of hydrolytic rancidity of fats.
- 11M.3.sl.TZ2.B1c: Explain whether the triglyceride in part (b) is a solid or a liquid at room temperature.
- 11M.3.sl.TZ2.B1d: Identify the type of reaction that occurs during the formation of a triglyceride.
- 11M.3.sl.TZ2.B1a: Identify the compounds X and Y. X: Y:
- 11M.3.sl.TZ2.B1b: Draw the structural formula of a triglyceride formed from one molecule each of octanoic acid,...
- 11M.3.sl.TZ2.F1a.ii: The hydrolysis of milk products is used in the making of cheese. State two conditions which...
- 12M.3.sl.TZ1.B1b: This energy comes mainly from the combustion of triglycerides. State the name of one other...
- 12M.3.sl.TZ1.F1b: Identify the types of nutrients A, B and C. A B C
- 12M.3.sl.TZ1.B1c: Explain why lipids have a higher energy content than carbohydrates.
- 12M.3.sl.TZ1.F2a: Rancidity can occur as a result of two separate processes. State these processes and explain...
- 12M.3.sl.TZ2.B3a: (i) Identify the major source of low-density lipoproteins. (ii) State the importance...
- 12M.3.sl.TZ2.B3b.i: Compare the chemical structures of linoleic acid, an omega-6 fatty acid, and linolenic acid,...
- 12M.3.sl.TZ2.F2a: State two major differences in their structures.
- 12M.3.sl.TZ2.F2c: Discuss two advantages and two disadvantages of converting oils into fats.
- 11N.3.sl.TZ0.F1b.ii: Deduce the structural formula of a triester formed from three long-chain carboxylic acid...
- 11N.3.sl.TZ0.F2a: State the meaning of the term rancidity as it applies to fats.
- 11N.3.sl.TZ0.F2b.i: Compare the two rancidity processes. Hydrolytic process: Oxidative process:

