| Date | May 2011 | Marks available | 1 | Reference code | 11M.3.sl.TZ2.B1 |
| Level | SL | Paper | 3 | Time zone | TZ2 |
| Command term | Draw | Question number | B1 | Adapted from | N/A |
Question
Triglycerides are one of three types of lipid found in the human body. The following equation represents the formation of a triglyceride.
X + 3RCOOH \( \rightleftharpoons \) triglyceride + 3Y
Identify the compounds X and Y.
X:
Y:
Draw the structural formula of a triglyceride formed from one molecule each of octanoic acid, lauric acid and stearic acid. The formulas of the acids are shown in Table 22 of the Data Booklet.
Explain whether the triglyceride in part (b) is a solid or a liquid at room temperature.
Identify the type of reaction that occurs during the formation of a triglyceride.
Explain why fats have a higher energy value per mole than carbohydrates.
Markscheme
X is glycerol/propane-1,2,3-triol/ \({\text{C}}{{\text{H}}_2}{\text{(OH)CH(OH)C}}{{\text{H}}_2}{\text{(OH)}}\);
Y is water/ \({{\text{H}}_2}{\text{O}}\);
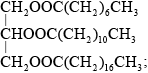
Accept the fatty acids in any order.
solid as contains (three) saturated/straight fatty acid chains;
can pack closer together;
have stronger London/dispersion/van der Waals’ forces between chains;
esterification / condensation;
fats contain less oxygen than carbohydrates / are in a less oxidised state (so more energy is released);
Examiners report
This question which was expected to be fairly straightforward proved to be rather tricky for candidates. In part (a) very few correctly identified glycerol in the formation of a triglyceride.
Drawing the structure of a triglyceride in (b) was challenging for many.
Some candidates explained very well why the triglyceride was a solid at room temperature, but others could only state that it was solid and were unclear of the reasons.
Only the best candidates could explain why fats have a higher energy value per mole than carbohydrates.

