| Date | May 2014 | Marks available | 3 | Reference code | 14M.3.sl.TZ2.5 |
| Level | SL | Paper | 3 | Time zone | TZ2 |
| Command term | Calculate and Define | Question number | 5 | Adapted from | N/A |
Question
Lipids are a group of naturally occurring largely non-polar biomolecules. The term iodine number is often used to characterize particular lipids.
(i) Define the term iodine number.
(ii) A sample containing \(1.12 \times {10^{ - 2}}\) mol of fatty acid was found to react with 8.50 g of iodine, \({{\text{I}}_{\text{2}}}\). Calculate the number of carbon-carbon double bonds present in the fatty acid, showing your working.
(i) Draw the structure of glycerol (propane-1,2,3-triol).
(ii) Glycerol can react with three molecules of lauric acid to form a triglyceride.
The structure of lauric acid is given in Table 22 of the Data Booklet. State the name of the functional group of the triglyceride and identify the other product formed.
Name of functional group of triglyceride:
Other product formed:
The hydrolysis of tristearin, whose structure is shown below, can be catalysed by the enzyme lipase.
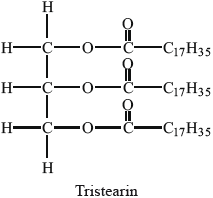
Successive hydrolysis of tristearin results in the formation of distearin and monostearin.
Deduce the structure of the diglyceride, distearin, and state the name of the other product formed from this reaction.
Structure of diglyceride, distearin:
Name of other product:
Explain why the metabolism of fats produces much more energy per gram than that of carbohydrates.
Markscheme
(i) mass (in g) of \({{\text{I}}_{\text{2}}}\) reacting with 100 g of fat/oil/substance/lipid;
Allow amount/number of mol of I2 reacting with 1 mol of fat/oil/substance/lipid.
(ii) \(\frac{{8.50}}{{253.4}}/\frac{{8.50}}{{254}}/3.35 \times {10^{ - 2}}{\text{ (mol)}}\);
\(\left( {\frac{{3.35 \times {{10}^{ - 2}}}}{{1.12 \times {{10}^{ - 2}}}}} \right) = 3\) (C=C);
OR
\(\frac{{8.50}}{{1.12}} \times {10^{ - 2}}/759\) (g \({{\text{I}}_{\text{2}}}\) react with one mol of fatty acid);
\(\left( {\frac{{759}}{{254}}} \right) = 3\) (C=C);
M2 can only be awarded if M1 is correct.
(i) 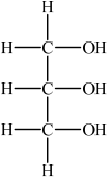 ;
;
Accept any correct representation.
(ii) Name of functional group of triglyceride:
ester
Allow triester.
Do not allow –COO–.
and
Other product formed:
water/\({{\text{H}}_{\text{2}}}{\text{O}}\)
Structure of diglyceride, distearin:
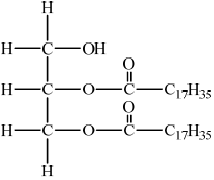 ;
;
Accept a structure with OH in middle also.
Name of other product:
stearic acid/octadecanoic acid / stearate/octadecanoate;
Name required.
Do not allow stearin.
fats have fewer oxygens than carbohydrates (of same molar mass) / fats less oxidized;
Allow converse statements for carbohydrates.
a larger change in carbon’s oxidation number occurs when fats are oxidized / more energy is used in breaking the bonds in carbohydrates than the bonds in fats;
Examiners report
(i) About half of the candidates could define iodine number correctly. Others had the correct idea but gave imprecise answers.
(ii) About half of the candidates obtained the number of moles of iodine that reacted and gained one mark. A smaller number of candidates were able to calculate the number of double bonds in the fatty acid.
(i) Almost all candidates were able to draw the structure of glycerol.
(ii) About half of the candidates recognized the functional group in a triglyceride as an ester, and more than half of the candidates recognized water as the other product formed.
This was a discriminating question. Only a few candidates were able to deduce the structure of the diglyceride and even fewer recognized the other product as stearic acid.
Many candidates obtained a mark for stating that fats contain less oxygen or are less oxidized than carbohydrates, but were unable to score the second mark.

