| Date | November 2010 | Marks available | 2 | Reference code | 10N.2.sl.TZ0.4 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Explain | Question number | 4 | Adapted from | N/A |
Question
Ethene, C2H4, and hydrazine, N2H4, are hydrides of adjacent elements in the periodic table.
The polarity of a molecule can be explained in terms of electronegativity.
The reaction between N2H4(aq) and HCl (aq) can be represented by the following equation.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{aq)}} + 2{\text{HCl(aq)}} \to {{\text{N}}_2}{\text{H}}_6^{2 + }({\text{aq)}} + 2{\text{C}}{{\text{l}}^ - }({\text{aq)}}\]
(i) Draw Lewis (electron dot) structures for \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{4}}}\) and \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}\) showing all valence electrons.
(ii) State and explain the H–C–H bond angle in ethene and the H–N–H bond angle in hydrazine.
(i) Define the term electronegativity.
(ii) Compare the relative polarities of the C–H bond in ethene and the N–H bond in hydrazine.
(iii) Hydrazine is a polar molecule and ethene is non-polar. Explain why ethene is non-polar.
The boiling point of hydrazine is much higher than that of ethene. Explain this difference in terms of the intermolecular forces in each compound.
Hydrazine is a valuable rocket fuel.
The equation for the reaction between hydrazine and oxygen is given below.
\[{{\text{N}}_2}{{\text{H}}_4}({\text{g)}} + {{\text{O}}_2}({\text{g)}} \to {{\text{N}}_2}({\text{g)}} + 2{{\text{H}}_2}{\text{O(g)}}\]
Use the bond enthalpy values from Table 10 of the Data Booklet to determine the enthalpy change for this reaction.
State the name of the product and identify the type of reaction which occurs between ethene and hydrogen chloride.
(i) Identify the type of reaction that occurs.
(ii) Predict the value of the H–N–H bond angle in \({{\text{N}}_{\text{2}}}{\text{H}}_6^{2 + }\).
Markscheme
(i) 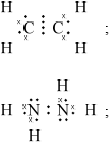
Accept x’s, dots or lines for electron pairs
(ii) H–C–H:
any angle between 118° and 122°;
due to three negative charge centres/electron domains/electron pairs;
H–N–H:
any angle between 104° and 108°;
due to four negative charge centres/electron domains/electron pairs;
extra repulsion due to lone electron pairs;
Do not allow ECF for wrong Lewis structures.
(i) (relative) measure of an atoms attraction for electrons;
in a covalent bond / shared pair;
(ii) C–H is less polar as C is less electronegative / N–H bond is more polar as N is more electronegative / difference in electronegativity is greater for N-H than C-H;
(iii) bond polarities cancel in \({{\text{C}}_2}{{\text{H}}_4}\) / OWTTE;
weaker van der Waals’/London/dispersion/intermolecular forces in ethene;
stronger (intermolecular) hydrogen bonding in hydrazine;
If no comparison between strengths then [1 max].
bonds broken: 4 N–H, N–N, O=O / \( + {\text{2220 (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
bonds formed: N\(\equiv\)N, 4O–H / \( - 2801{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
\( - 581{\text{ (kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
chloroethane;
(electrophilic) addition;
Do not accept free radical/nucleophilic addition.
(i) acid-base/neutralization;
(ii) 109°/109.5°;
Examiners report
This was a popular question and was answered quite successfully. The Lewis structure for ethene was given correctly by the great majority of the candidates, but that of hydrazine by only about half of them. Incorrect answers had double bonds appearing between the 2 nitrogen atoms and lone pairs on nitrogen atoms not shown. Those who could draw the correct structure in (i) gave the correct bond angle, but the explanation was not given correctly by many. Only very few scored the five marks as many failed to mention the extra repulsion of the lone pair.
The definition of electronegativity was not well known and many forgot to mention covalent bond or got confused with ionization and electron affinity and talked about a mole of gaseous atoms.
In part (c) most knew that hydrogen bonding in hydrazine was stronger than the van der Waals’ forces in ethene and explained its higher boiling point. However, some candidates described hydrogen bonding as the bond between N and H in the molecule, and some omitted a comparison of the relative strengths.
The calculation for the enthalpy change produced some completely correct calculations but many candidates lost marks here for using the wrong bond energies, although ECF was applied to the structures drawn in part (a).
In (e) ‘addition’ was correctly identified as the reaction type by most but when asked in (f) to identify the final reaction type few recognised it as an acid-base reaction, however, the bond angle was given correctly by many.
In (e) ‘addition’ was correctly identified as the reaction type by most but when asked in (f) to identify the final reaction type few recognised it as an acid-base reaction, however, the bond angle was given correctly by many.

