| Date | November 2014 | Marks available | 1 | Reference code | 14N.2.hl.TZ0.3 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Sketch | Question number | 3 | Adapted from | N/A |
Question
The vapour pressure of water changes with temperature according to the graph below.
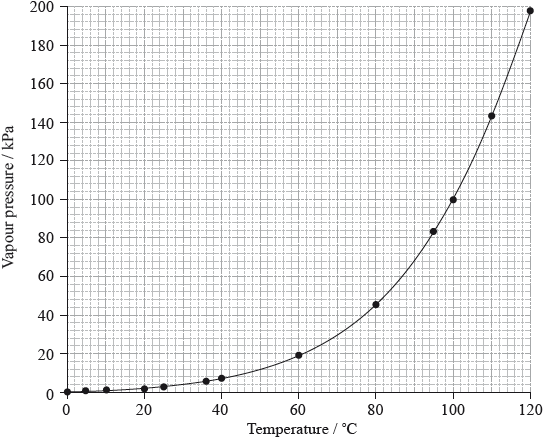
A liquid boils when its vapour pressure equals atmospheric pressure. Determine the boiling point of water on a mountaintop on a day when the atmospheric pressure is 60.0 kPa.
Sketch another curve on the axes above to show how the vapour pressure of a liquid that has weaker intermolecular forces than water, such as bromine, changes with temperature.
(i) A sample of liquid bromine was left in a closed conical (Erlenmeyer) flask at 298 K and allowed to reach a state of equilibrium. State an observation that indicates that equilibrium was reached.
(ii) The temperature of the closed flask was increased and the system was allowed to reach a new equilibrium. Compare the equilibrium formed at the new temperature with the equilibrium at the original temperature on the molecular level.
Markscheme
87 (°C);
Accept boiling points in the range 86–88 °C.
similar shape above current curve / steeper than current curve;
Do not penalize if curves meet at 0 °C.
(i) (intensity of) colour of vapour is constant;
Accept volume/level of liquid is constant.
Allow pressure is constant.
(ii) more (molecules in the) vapour / fewer molecules in the liquid at new equilibrium / OWTTE;
molecules have more energy/move faster/collide more frequently at the new temperature / OWTTE;
rates of evaporation and condensation are higher at the new temperature;
in both flasks the rates of evaporation and condensation are equal;
Accept converse points for the flask at lower temperature for M1, M2 and M3.
Examiners report
A very well answered question.
Most candidates presented a curve that was steeper than the water vapour curve gaining the mark. However, most of the candidates started from the same vapour pressure as water at 0 °C which was not penalized. Very few candidates drew an accurate curve.
(i) This question was not well answered. Only a few candidates were able to give an appropriate observation. Many candidates could state the characteristics of a system in equilibrium but did not apply their knowledge to state an observation.
(ii) Only a few candidates gave adequate explanations gaining two marks. Many obtained one mark for saying that more molecules will be in the gaseous state. The reference to “molecular level” often went unnoticed.

