| Date | May 2014 | Marks available | 1 | Reference code | 14M.2.sl.TZ2.4 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Outline | Question number | 4 | Adapted from | N/A |
Question
Group 7 of the periodic table contains a number of reactive elements such as chlorine, bromine and iodine.
Bleaches in which chlorine is the active ingredient are the most common, although some environmental groups have concerns about their use. In aqueous chlorine the equilibrium below produces chloric(I) acid (hypochlorous acid), HOCl, the active bleach.
\[{\text{C}}{{\text{l}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{HOCl (aq)}} + {{\text{H}}^ + }{\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}\]
Aqueous sodium chlorate(I), NaOCl, the most common active ingredient in chlorine based bleaches, oxidizes coloured materials to colourless products while being reduced to the chloride ion. It will also oxidize sulfur dioxide to the sulfate ion.
(i) Describe the colour change that occurs when aqueous chlorine is added to aqueous sodium bromide.
(ii) Outline, with the help of a chemical equation, why this reaction occurs.
The colour change in the reaction between aqueous chlorine and aqueous sodium iodide is very similar, but it differs with an excess of aqueous chlorine. Describe the appearance of the reaction mixture when excess aqueous chlorine has been added to aqueous sodium iodide.
Chloric(I) acid is a weak acid, but hydrochloric acid is a strong acid. Outline how this is indicated in the equation above.
State a balanced equation for the reaction of chloric(I) acid with water.
Outline, in terms of the equilibrium above, why it is dangerous to use an acidic toilet cleaner in combination with this kind of bleach.
Suggest why a covalent molecule, such as chloric(I) acid, is readily soluble in water.
Draw the Lewis (electron dot) structure of chloric(I) acid.
Predict the H–O–Cl bond angle in this molecule and explain this in terms of the valence shell electron pair repulsion (VSEPR) theory.
(i) Deduce the coefficients required to balance the half-equations given below.
___ \({\text{Cl}}{{\text{O}}^ - } + \) ___ \({{\text{H}}^ + } + \) ___ \({{\text{e}}^ - } \rightleftharpoons \) ___ \({{\text{H}}_2}{\text{O}} + \) ___ \({\text{C}}{{\text{l}}^ - }\)
___ \({\text{SO}}_4^{2 - }\) ___ \({{\text{H}}^ + } + \) ___ \({{\text{e}}^ - } \rightleftharpoons \) ___ \({\text{S}}{{\text{O}}_2} + \) ___ \({{\text{H}}_2}{\text{O}}\)
(ii) State the initial and final oxidation numbers of both chlorine and sulfur in the equations in part (i).

(iii) Use the half-equations to deduce the balanced equation for the reaction between the chlorate(I) ion and sulfur dioxide.
Markscheme
(i) from (pale) green/colourless to yellow/orange/brown;
Initial colour must be stated.
Do not accept “clear/transparent” instead of “colourless”.
(ii) chlorine more reactive/more powerful oxidizing agent (than bromine);
Accept opposite statements for bromine.
Accept “chloride ion a weaker reducing agent” / “bromide ion a stronger reducing agent”.
Accept “chlorine more electronegative than bromine”.
\({\text{C}}{{\text{l}}_2}{\text{(aq)}} + {\text{2NaBr(aq)}} \to {\text{B}}{{\text{r}}_2}{\text{(aq)}} + {\text{2NaCl(aq)}}\) /
\({\text{C}}{{\text{l}}_2}{\text{(aq)}} + {\text{2B}}{{\text{r}}^ - }{\text{(aq)}} \to {\text{B}}{{\text{r}}_2}{\text{(aq)}} + {\text{2C}}{{\text{l}}^ - }{\text{(aq)}}\);
Ignore state symbols.
Do not accept with equilibrium sign.
solid (in a colourless solution);
Accept “dark brown solution”.
chloric(I) acid (shown as) a molecule/molecular, but hydrochloric acid (shown as being) split into ions / OWTTE;
Accept “chloric(I) acid is partially dissociated and hydrochloric acid is fully dissociated”.
Reference needed to both acids for mark.
\({\text{HOCl(aq)}} \rightleftharpoons {{\text{H}}^ + }{\text{(aq)}} + {\text{Cl}}{{\text{O}}^ - }{\text{(aq)}}/{\text{HOCl(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {{\text{H}}_3}{{\text{O}}^ + }{\text{(aq)}} + {\text{Cl}}{{\text{O}}^ - }{\text{(aq)}}\);
Equilibrium sign required for the mark.
Ignore state symbols.
acid displaces the equilibrium to the left (to form chlorine);
chlorine is toxic/poisonous/harmful/lung irritant;
Accept answers that refer to the (c) (ii) equilibrium.
chloric(I) acid has –OH group / hydrogen attached to a very electronegative atom;
Accept polar molecule.
can form hydrogen bonds to water;
hydrogen bonding to water increases its solubility;
(as a weak acid it is) in equilibrium with ions;
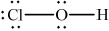 ;
;
Accept lines, dots or crosses to represent electron pairs.
\( \sim\)104°;
Accept values between 102° and 106°.
four electron pairs/regions of high electron density around O atom / electron pairs/regions of high electron density tetrahedrally arranged and two lone/non-bonding electron pairs on O atom;
Accept Lewis structure with two lone pairs on O and two angular bond pairs if given here as equivalent to M2.
lone pair–bonding pair repulsion greater than bonding pair–bonding pair repulsion;
(i) \({\text{(1) Cl}}{{\text{O}}^ - } + \) 2\(\,{{\text{H}}^ + } + \) 2\(\,{{\text{e}}^ - } \rightleftharpoons {\text{(1) }}{{\text{H}}_2}{\text{O}} + {\text{(1) C}}{{\text{l}}^ - }\);
\({\text{(1) SO}}_4^{2 - } + \) 4\(\,{{\text{H}}^ + } + \) 2\(\,{{\text{e}}^ - } \rightleftharpoons {\text{(1) S}}{{\text{O}}_2} + \) 2\(\,{{\text{H}}_2}{\text{O}}\);
(ii) Award [2] for all correct, [1] for 2 or 3 correct.

Remember to apply ECF from previous equations.
Penalize incorrect notation (eg, 4 or 4+ rather than +4) once only, so award [1] for a fully correct answer in an incorrect format.
(iii) \({\text{Cl}}{{\text{O}}^ - }{\text{(aq)}} + {\text{S}}{{\text{O}}_2}{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{SO}}_4^{2 - }{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{C}}{{\text{l}}^ - }{\text{(aq)}}\)
correct reactants and products;
balancing and cancelling \({{\text{e}}^ - }\), \({{\text{H}}^ + }\) and \({{\text{H}}_{\text{2}}}{\text{O}}\);
Ignore state symbols.
Do not penalize equilibrium sign.
Examiners report
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.
This was the least popular and the least successfully answered question on the paper. Many were unable to describe the colour change required in (a)(i) though more could give an appropriate equation and explain why the reaction occurred in terms of electronegativity. (b) was essentially a “dead” mark and perhaps was out of place on a SL paper. Many students seemed to be aware of the difference between strong and weak acids, but few could use this to answer (c)(i), and many were unable to write an equation for its reaction in water. The more able candidates realised that acids would affect the position of the equilibrium and a number recognized that the toxic gas chlorine would be a product. Many students identified hydrogen bonding from the –OH group as being the reason for the solubility of HOCl. Most were able to give the Lewis (electron dot) structure of chloric(I) acid, but few were able to give a detailed explanation of its bond angle, with only a minority referring to electron domains. In part (d) very few students could write, or combine, appropriate half equations, even though the reactants and products were given, though many could deduce the oxidation numbers of the species in the equations. Some marks were unfortunately lost as candidates omitted the sign.

