| Date | November 2012 | Marks available | 4 | Reference code | 12N.2.sl.TZ0.4 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Deduce, Explain, and Identify | Question number | 4 | Adapted from | N/A |
Question
Lithium and boron are elements in period 2 of the periodic table. Lithium occurs in group 1 (the alkali metals) and boron occurs in group 3. Isotopes exist for both elements.
Every element has its own unique line emission spectrum.
(i) Define the terms atomic number, mass number and isotopes of an element.
Atomic number:
Mass number:
Isotopes of an element:
(ii) Distinguish between the terms group and period.
(iii) Deduce the electron arrangements of the lithium ion, \({\text{L}}{{\text{i}}^ + }\), and the boron atom, B.
\({\text{L}}{{\text{i}}^ + }\):
B:
(iv) Naturally occurring boron exists as two isotopes with mass numbers of 10 and 11. Calculate the percentage abundance of the lighter isotope, using this information and the relative atomic mass of boron in Table 5 of the Data Booklet.
v) Lithium exists as two isotopes with mass numbers of 6 and 7. Deduce the number of protons, electrons and neutrons for each isotope.
(i) Distinguish between a continuous spectrum and a line spectrum.
(ii) Draw a diagram to show the electron transitions between energy levels in a hydrogen atom that are responsible for the two series of lines in the ultraviolet and visible regions of the spectrum. Label your diagram to show three transitions for each series.
(i) Explain why metals are good conductors of electricity and why they are malleable.
(ii) Iron is described as a transition metal. Identify the two most common ions of iron.
iii) Deduce the chemical formulas of lithium oxide and iron(II) oxide.
Lithium oxide:
Iron(II) oxide:
Markscheme
(i) Atomic number:
number of protons (in nucleus/atom);
Mass number:
(sum of) number of protons and neutrons (in nucleus/atom);
Isotopes of an element:
atoms of same element / atoms with same number of protons/atomic number/Z but different number of neutrons/mass number/A;
Penalize once only use of the term element in the three definitions, for example, number of protons in an element or number of protons and neutrons in an element or element with the same atomic number but different mass number.
(ii) Group: (elements in vertical) columns in periodic table and Period: (elements in horizontal) rows in periodic table;
Allow elements in same group have similar chemical properties and within a period, atoms have same number of shells/energy levels (but number of electrons in valence/outer shell increases).
Allow groups distributed vertically and periods distributed horizontally / OWTTE.
Allow group number gives number of valence/outer shell electrons (for maingroup elements) and period gives same number of shells/energy levels.
(iii) Li+: 2/1s2;
B: 2,3/1s22s22p1;
(iv) correct mathematical expression set-up \({\text{(e.g. }}\left( {\frac{x}{{100}}} \right)(10) + \left[ {\frac{{(100 - x)}}{{(100)}}} \right](11) = 10.81)\);
19%;
Award [2] for correct final answer.
(v) 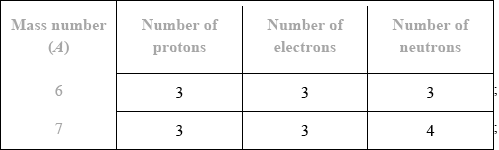
Award [1 max] for correct number of neutrons for both isotopes if numbers of protons or electrons is not given.
Award [1 max] for correct number of protons and electrons for both isotopes if number of neutrons is not given or if numbers of neutrons are incorrect.
(i) Continuous spectrum: radiation spread over all wavelengths/frequencies/energies/colours / OWTTE;
Line spectrum: radiation (absorbed/emitted) at certain/specific wavelengths/frequencies/energies/colours / OWTTE;
Allow series of (separate/discrete) lines which converge/get closer together at high energy / OWTTE.
(ii) 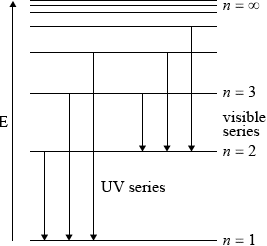
showing y-axis labelled as energy/E or labelling at least two energy levels
(\(n = 1\), \(n = 2\) etc. but not for \(n = 0\));
showing energy levels converging;
showing jumps to \(n = 1\) for ultraviolet series;
showing jumps to \(n = 2\) for visible series;
UV and visible must be labelled.
(i) metals have delocalized electrons / sea of electrons which are mobile/can move / OWTTE;
layers/positive ions/cations/atoms slide past/over each other / OWTTE;
Do not accept nuclei for M2.
(ii) Fe2+ and Fe3+ ;
(iii) Lithium oxide: Li2O and Iron(II) oxide: FeO;
Examiners report
Many candidates defined the atomic number, mass number and isotopes correctly although the weaker candidates incorrectly used the term element instead of atom and others defined mass number in terms of molar mass instead of sum of protons and neutrons in the nucleus. Distinguishing between a group and a period and deducing the electron arrangements of Li+ and boron was handled well by majority of candidates. Many candidates struggled to calculate the percentage abundance of the lighter isotope whereas in part (v), most candidates correctly deduced the number of protons, neutrons and electrons in the two isotopes of lithium.
Distinguishing between a continuous and line spectrum in part (b) proved difficult for many candidates. Similarly, drawing a diagram to show the electron transitions between energy levels in a hydrogen atom was challenging for many candidates. Common errors seen were: starting incorrectly at \(n = 0\), not showing convergence or mixed up between the ultraviolet and visible lines.
In Part (c), although the explanation of why metals are good conductors of electricity was answered well, some candidates did not refer to delocalized or sea of electrons. Explanation of why metals are malleable proved to be difficult for many candidates. Identifying the two most common ions of iron and deducing chemical formulas was correctly answered by majority of the candidates.

