| Date | May 2015 | Marks available | 2 | Reference code | 15M.2.sl.TZ2.6 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Explain | Question number | 6 | Adapted from | N/A |
Question
Draw the Lewis (electron dot) structure of chloromethane.
Predict the shape of the chloromethane molecule and the H–C–H bond angle.
Shape:
Bond angle:
Explain why chloromethane is a polar molecule.
Methanol has a lower molar mass than chloromethane. Explain why the boiling point of methanol is higher than that of chloromethane.
State the equation for the reaction between potassium and chlorine.
Outline the nature of the metallic bonding present in potassium.
Describe the covalent bond present in the chlorine molecule and how it is formed.
Describe the ionic bonding present in potassium chloride and how the ions are formed.
Potassium also reacts with water to form hydrogen gas. Determine the volume, in \({\text{c}}{{\text{m}}^{\text{3}}}\), of hydrogen gas that could theoretically be produced at 273 K and \(1.01 \times {10^5}{\text{ Pa}}\) when 0.0587 g of potassium reacts with excess water.
Identify the acid-base character of the oxides of each of the elements from sodium to chlorine in period 3.
State the equations for the separate reactions of sodium oxide and phosphorus(V) oxide with water.
Markscheme
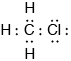 ;
;
Accept any combination of lines, dots or crosses to represent electron pairs.
Shape: tetrahedral;
Bond angle: accept any value in the range: 108° to 111°;
(Literature value is 108.2°).
Cl is more electronegative than C / C–Cl bond polar;
bond dipoles do not cancel / asymmetric distribution of electron cloud / (resultant) net dipole moment (from vectorial addition of bond dipoles) going in direction of C–Cl axis / OWTTE;
hydrogen bonding in methanol;
stronger than dipole-dipole/van der Waals’ attractions/forces in chloromethane;
Accept converse argument.
\({\text{2K(s)}} + {\text{C}}{{\text{l}}_2}{\text{(g)}} \to {\text{2KCl(s)}}\);
Ignore state symbols.
(electrostatic) attraction between lattice of cations/positive ions and delocalized electrons;
(electrostatic) attraction between positively charged nuclei and a pair of electrons;
formed as a result of electron sharing;
(electrostatic) attraction between positive and negative ions/oppositely charged ions/cations and anions;
formed as a result of transfer of an electron from a K atom to a Cl atom / OWTTE;
amount of potassium \( = \left( {\frac{{0.0587}}{{39.10}} = } \right){\text{ }}1.5 \times {10^{ - 3}}{\text{ (mol)}}\);
\({\text{2K}} + {\text{2}}{{\text{H}}_2}{\text{O}} \to {\text{2KO}} + {{\text{H}}_2}\) / amount of hydrogen \( = 7.50 \times {10^{ - 4}}{\text{ (mol)}}\);
volume of hydrogen \( = (7.50 \times {10^{ - 4}} \times 22.4 \times 1000 = ){\text{ }}16.8{\text{ (c}}{{\text{m}}^3}{\text{)}}\);
Accept calculation of volume of hydrogen using PV = nRT (answer is 16.9 cm3).
Award [3] for correct final answer.
Na, Mg (oxides): basic
Al (oxide): amphoteric
Do not accept amphiprotic.
Si to Cl (oxides): acidic
Award [2] for all three listed sets correct.
Award [1] for one or two listed sets correct.
Award [1] for stating oxides become more acidic towards right/Cl or more basic towards left/Na.
Do not penalize if reference is to Ar instead of Cl.
Do not penalize for incorrect formulas of oxides.
\({\text{N}}{{\text{a}}_2}{\text{O(s)}} + {{\text{H}}_2}{\text{O(l)}} \to {\text{2NaOH(aq)}}\);
\({{\text{P}}_4}{{\text{O}}_{10}}{\text{(s)}} + {\text{6}}{{\text{H}}_2}{\text{O(l)}} \to {\text{4}}{{\text{H}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}}\);
Ignore state symbols.
Accept P2O5(s) + 3H2O(l) \( \to \) 2H3PO4(aq).
Do not award marks if incorrect formulas of the oxides are used.
Examiners report
Probably the least popular option. The drawing of the diagram of chloromethane was generally excellent, as was the prediction/recall of the shape and bond angle. With the reasons for polarity, the concept of bond polarity was well understood, but the idea of asymmetry resulting in a dipole was less clearly appreciated. The construction of the chemical equation was disappointing, as was the description of the three types of bonding, very often missing the important point, in that they are attractions. With the calculation of volume of hydrogen, it was quite rare to get a fully correct answer. The biggest error was to use an incorrect value for the number of moles of hydrogen in the equation \(pV = nRT\), by failing to halve the moles of hydrogen. The use of \(pV = nRT\) also caused problems with units. The acid base nature of oxides of a period were generally well known. In contrast, the construction or recall of correct chemical equations for the reaction with water was a weakness.
Probably the least popular option. The drawing of the diagram of chloromethane was generally excellent, as was the prediction/recall of the shape and bond angle. With the reasons for polarity, the concept of bond polarity was well understood, but the idea of asymmetry resulting in a dipole was less clearly appreciated. The construction of the chemical equation was disappointing, as was the description of the three types of bonding, very often missing the important point, in that they are attractions. With the calculation of volume of hydrogen, it was quite rare to get a fully correct answer. The biggest error was to use an incorrect value for the number of moles of hydrogen in the equation \(pV = nRT\), by failing to halve the moles of hydrogen. The use of \(pV = nRT\) also caused problems with units. The acid base nature of oxides of a period were generally well known. In contrast, the construction or recall of correct chemical equations for the reaction with water was a weakness.
Probably the least popular option. The drawing of the diagram of chloromethane was generally excellent, as was the prediction/recall of the shape and bond angle. With the reasons for polarity, the concept of bond polarity was well understood, but the idea of asymmetry resulting in a dipole was less clearly appreciated. The construction of the chemical equation was disappointing, as was the description of the three types of bonding, very often missing the important point, in that they are attractions. With the calculation of volume of hydrogen, it was quite rare to get a fully correct answer. The biggest error was to use an incorrect value for the number of moles of hydrogen in the equation \(pV = nRT\), by failing to halve the moles of hydrogen. The use of \(pV = nRT\) also caused problems with units. The acid base nature of oxides of a period were generally well known. In contrast, the construction or recall of correct chemical equations for the reaction with water was a weakness.
Probably the least popular option. The drawing of the diagram of chloromethane was generally excellent, as was the prediction/recall of the shape and bond angle. With the reasons for polarity, the concept of bond polarity was well understood, but the idea of asymmetry resulting in a dipole was less clearly appreciated. The construction of the chemical equation was disappointing, as was the description of the three types of bonding, very often missing the important point, in that they are attractions. With the calculation of volume of hydrogen, it was quite rare to get a fully correct answer. The biggest error was to use an incorrect value for the number of moles of hydrogen in the equation \(pV = nRT\), by failing to halve the moles of hydrogen. The use of \(pV = nRT\) also caused problems with units. The acid base nature of oxides of a period were generally well known. In contrast, the construction or recall of correct chemical equations for the reaction with water was a weakness.
Probably the least popular option. The drawing of the diagram of chloromethane was generally excellent, as was the prediction/recall of the shape and bond angle. With the reasons for polarity, the concept of bond polarity was well understood, but the idea of asymmetry resulting in a dipole was less clearly appreciated. The construction of the chemical equation was disappointing, as was the description of the three types of bonding, very often missing the important point, in that they are attractions. With the calculation of volume of hydrogen, it was quite rare to get a fully correct answer. The biggest error was to use an incorrect value for the number of moles of hydrogen in the equation \(pV = nRT\), by failing to halve the moles of hydrogen. The use of \(pV = nRT\) also caused problems with units. The acid base nature of oxides of a period were generally well known. In contrast, the construction or recall of correct chemical equations for the reaction with water was a weakness.
Probably the least popular option. The drawing of the diagram of chloromethane was generally excellent, as was the prediction/recall of the shape and bond angle. With the reasons for polarity, the concept of bond polarity was well understood, but the idea of asymmetry resulting in a dipole was less clearly appreciated. The construction of the chemical equation was disappointing, as was the description of the three types of bonding, very often missing the important point, in that they are attractions. With the calculation of volume of hydrogen, it was quite rare to get a fully correct answer. The biggest error was to use an incorrect value for the number of moles of hydrogen in the equation \(pV = nRT\), by failing to halve the moles of hydrogen. The use of \(pV = nRT\) also caused problems with units. The acid base nature of oxides of a period were generally well known. In contrast, the construction or recall of correct chemical equations for the reaction with water was a weakness.
Probably the least popular option. The drawing of the diagram of chloromethane was generally excellent, as was the prediction/recall of the shape and bond angle. With the reasons for polarity, the concept of bond polarity was well understood, but the idea of asymmetry resulting in a dipole was less clearly appreciated. The construction of the chemical equation was disappointing, as was the description of the three types of bonding, very often missing the important point, in that they are attractions. With the calculation of volume of hydrogen, it was quite rare to get a fully correct answer. The biggest error was to use an incorrect value for the number of moles of hydrogen in the equation \(pV = nRT\), by failing to halve the moles of hydrogen. The use of \(pV = nRT\) also caused problems with units. The acid base nature of oxides of a period were generally well known. In contrast, the construction or recall of correct chemical equations for the reaction with water was a weakness.
Probably the least popular option. The drawing of the diagram of chloromethane was generally excellent, as was the prediction/recall of the shape and bond angle. With the reasons for polarity, the concept of bond polarity was well understood, but the idea of asymmetry resulting in a dipole was less clearly appreciated. The construction of the chemical equation was disappointing, as was the description of the three types of bonding, very often missing the important point, in that they are attractions. With the calculation of volume of hydrogen, it was quite rare to get a fully correct answer. The biggest error was to use an incorrect value for the number of moles of hydrogen in the equation \(pV = nRT\), by failing to halve the moles of hydrogen. The use of \(pV = nRT\) also caused problems with units. The acid base nature of oxides of a period were generally well known. In contrast, the construction or recall of correct chemical equations for the reaction with water was a weakness.
Probably the least popular option. The drawing of the diagram of chloromethane was generally excellent, as was the prediction/recall of the shape and bond angle. With the reasons for polarity, the concept of bond polarity was well understood, but the idea of asymmetry resulting in a dipole was less clearly appreciated. The construction of the chemical equation was disappointing, as was the description of the three types of bonding, very often missing the important point, in that they are attractions. With the calculation of volume of hydrogen, it was quite rare to get a fully correct answer. The biggest error was to use an incorrect value for the number of moles of hydrogen in the equation \(pV = nRT\), by failing to halve the moles of hydrogen. The use of \(pV = nRT\) also caused problems with units. The acid base nature of oxides of a period were generally well known. In contrast, the construction or recall of correct chemical equations for the reaction with water was a weakness.
Probably the least popular option. The drawing of the diagram of chloromethane was generally excellent, as was the prediction/recall of the shape and bond angle. With the reasons for polarity, the concept of bond polarity was well understood, but the idea of asymmetry resulting in a dipole was less clearly appreciated. The construction of the chemical equation was disappointing, as was the description of the three types of bonding, very often missing the important point, in that they are attractions. With the calculation of volume of hydrogen, it was quite rare to get a fully correct answer. The biggest error was to use an incorrect value for the number of moles of hydrogen in the equation \(pV = nRT\), by failing to halve the moles of hydrogen. The use of \(pV = nRT\) also caused problems with units. The acid base nature of oxides of a period were generally well known. In contrast, the construction or recall of correct chemical equations for the reaction with water was a weakness.
Probably the least popular option. The drawing of the diagram of chloromethane was generally excellent, as was the prediction/recall of the shape and bond angle. With the reasons for polarity, the concept of bond polarity was well understood, but the idea of asymmetry resulting in a dipole was less clearly appreciated. The construction of the chemical equation was disappointing, as was the description of the three types of bonding, very often missing the important point, in that they are attractions. With the calculation of volume of hydrogen, it was quite rare to get a fully correct answer. The biggest error was to use an incorrect value for the number of moles of hydrogen in the equation \(pV = nRT\), by failing to halve the moles of hydrogen. The use of \(pV = nRT\) also caused problems with units. The acid base nature of oxides of a period were generally well known. In contrast, the construction or recall of correct chemical equations for the reaction with water was a weakness.

