| Date | November 2011 | Marks available | 1 | Reference code | 11N.1.hl.TZ0.24 |
| Level | HL | Paper | 1 | Time zone | TZ0 |
| Command term | Identify | Question number | 24 | Adapted from | N/A |
Question
Four identical sealed containers are prepared each containing \({\text{10 c}}{{\text{m}}^{\text{3}}}\) of an organic compound and at the temperature shown below. Which container will have the highest vapour pressure?
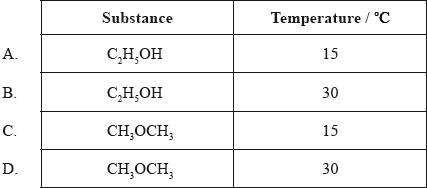
Markscheme
D
Examiners report
In this question, candidates were asked to identify the container with the highest vapour pressure from a list of two substances and related temperatures. One of the substances was \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OC}}{{\text{H}}_{\text{3}}}\), methoxymethane. One respondent stated that ethers are off-syllabus. This is an important point that has been addressed before in a number of subject reports. It is true to say that in the current programme candidates are not required to either identify ethers as a formal functional group or to name them, applying IUPAC rules. However, it should be noted that ethers are referred to in the TN corresponding to AS 4.3.2 in relation to the difference in boiling point between ethanol and methoxymethane, with regard to hydrogen bonding considerations. Hence, it is perfectly valid for methoxymethane to be cited in this particular question on vapour pressure.
Syllabus sections
- 17N.3.sl.TZ0.9c: Explain how the inclusion of carbohydrates in plastics makes them biodegradable.
- 17N.3.sl.TZ0.7b.i: Describe how the structures of LDPE and HDPE affect one mechanical property of the plastics.
- 17N.3.sl.TZ0.10a: Explain, at the molecular level, why vitamin D is soluble in fats. Use section 35 of the data...
- 17N.2.sl.TZ0.2b: Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
- 17N.1.sl.TZ0.11: Which of the following series shows increasing hydrogen bonding with water? A. Propane <...
- 17N.3.hl.TZ0.7c: Identify the type of intermolecular bonding that is responsible for Kevlar®’s strength.
- 17N.2.hl.TZ0.3b: Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group...
- 17M.3.sl.TZ2.16a: Explain why diamorphine passes more readily than morphine through the blood-brain barrier.
- 17M.3.sl.TZ2.11: Suggest, in terms of its structure, why vitamin D is fat-soluble using section 35 of the data...
- 17M.3.sl.TZ2.8a: Explain which one of these fatty acids has the highest boiling point.
- 17M.2.sl.TZ2.4a.ii: State why hydrazine has a higher boiling point than dinitrogen tetraoxide.
- 17M.1.sl.TZ2.10: Which bonds cause the boiling point of water to be significantly greater than that of...
- 17M.3.sl.TZ1.19a: Suggest why aspirin is slightly soluble in water. Refer to section 37 of the data booklet.
- 17M.3.sl.TZ1.13d: The solubility of a vitamin depends on its structure. Identify the vitamin given in section...
- 17M.3.sl.TZ1.11b.i: The drain pipe of a kitchen sink can become clogged by fatty acids, such as linoleic acid,...
- 17M.3.sl.TZ1.10b.ii: Explain why the difference in their structures affects their melting points.
- 17M.2.sl.TZ1.2e.ii: A chloride of titanium, TiCl4, melts at 248 K. Suggest why the melting point is so much lower...
- 17M.1.sl.TZ1.24: What is the order of increasing boiling point? A. C4H10 < CH3COOH < CH3CH2CHO <...
- 17M.1.sl.TZ1.12: Which correctly states the strongest intermolecular forces in the compounds below?
- 17M.1.sl.TZ1.9: A substance has the following properties: What is the most probable structure of this...
- 16N.3.hl.TZ0.22c: (i) Uranium hexafluoride, UF6, is used in the uranium enrichment process that produces fuel...
- 16N.3.sl.TZ0.18c: Justify the conclusion that recrystallization increased the purity of the product, by...
- 16N.3.sl.TZ0.18b: Suggest why isolation of the crude product involved the addition of ice-cold water.
- 16N.3.sl.TZ0.8b: The table below shows average figures for the percentage fatty acid composition of some...
- 16N.2.sl.TZ0.1c: Explain why the boiling point of ethane-1,2-diol is significantly greater than that of ethene.
- 16N.1.sl.TZ0.11: Between which pair of molecules can hydrogen bonding occur? A. CH4 and H2OB. CH3OCH3 and...
- 16M.2.hl.TZ0.1a: (i) Draw a Lewis (electron dot) structure of phosphine. (ii) State the hybridization of the...
- 16M.2.sl.TZ0.1a: (i) Draw a Lewis (electron dot) structure of phosphine. (ii) Outline whether you expect the...
- 16M.1.sl.TZ0.12: Which of the following are van der Waals’ forces? I. Dipole-dipole forcesII. Hydrogen...
- 15M.1.hl.TZ2.11: Which correctly lists butane \({\text{(}}{M_{\text{r}}} = {\text{58)}}\), propanone...
- 15M.2.hl.TZ1.1c: Identify the strongest intermolecular force in solid ethanedioic acid.
- 15M.2.hl.TZ1.2b.ii: Predict whether phosphorus(V) oxide and sodium oxide conduct electricity in their solid and...
- 15M.2.hl.TZ1.2b.i: Explain why the melting point of phosphorus(V) oxide is lower than that of sodium oxide in...
- 15M.2.hl.TZ2.10f: Explain why but-2-ene is more volatile than compound C.
- 15M.1.sl.TZ1.13: The following compounds have similar molar...
- 15M.3.hl.TZ2.28b.iii: Deduce, giving a reason, whether carotenoids are water-soluble or fat-soluble.
- 15M.1.sl.TZ1.12: Which forces are present between molecules of carbon dioxide in the solid state? A. ...
- 15M.2.sl.TZ1.1d: Identify the strongest intermolecular force in solid ethanedioic acid.
- 15M.2.sl.TZ1.7b: \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) exists as three isomers. Identify the...
- 15M.2.sl.TZ2.6a.iv: Methanol has a lower molar mass than chloromethane. Explain why the boiling point of methanol...
- 15M.2.sl.TZ2.7f: Explain why but-2-ene is more volatile than compound C,...
- 14M.1.hl.TZ2.24: Which combination of properties is correct?
- 14M.2.hl.TZ1.5c: (i) Explain why the boiling point of HF is much higher than the boiling points of the...
- 14M.2.hl.TZ2.1h: Suggest one other reason why using water as a solvent would make the experiment less successful.
- 14M.2.hl.TZ2.5b.i: Chloric(I) acid is a weak acid, but hydrochloric acid is a strong acid. Outline how this is...
- 14M.2.hl.TZ1.7b: (i) Compare the two liquids in terms of their boiling points, enthalpies of vaporization...
- 14M.2.hl.TZ2.1g: Propanone is used as the solvent because one compound involved in the equilibrium is...
- 14M.2.hl.TZ2.5b.ii: State a balanced equation for the reaction of chloric(I) acid with water.
- 14M.2.hl.TZ2.5b.iii: Outline, in terms of the equilibrium in aqueous chlorine, why it is dangerous to use an...
- 14M.2.hl.TZ2.5b.iv: Suggest why a covalent molecule, such as chloric(I) acid, is readily soluble in water.
- 14M.1.sl.TZ1.12: What is the correct order of increasing boiling point? A. ...
- 14M.2.sl.TZ1.4a: Deduce the order of increasing solubility in water of the three compounds.
- 14M.2.sl.TZ1.4b: Explain your reasoning.
- 14M.2.sl.TZ1.5c: (i) Explain why the boiling point of HF is much higher than the boiling points of the...
- 14M.2.sl.TZ2.1j: Propanone is used as the solvent because one compound involved in the equilibrium is...
- 14M.2.sl.TZ2.1k: Suggest one other reason why using water as a solvent would make the experiment less successful.
- 14M.2.sl.TZ2.4c.i: Chloric(I) acid is a weak acid, but hydrochloric acid is a strong acid. Outline how this is...
- 14M.2.sl.TZ2.4c.ii: State a balanced equation for the reaction of chloric(I) acid with water.
- 14M.2.sl.TZ2.4c.iii: Outline, in terms of the equilibrium above, why it is dangerous to use an acidic toilet...
- 14M.2.sl.TZ2.4c.iv: Suggest why a covalent molecule, such as chloric(I) acid, is readily soluble in water.
- 14M.3.sl.TZ1.18c: Explain why the triglyceride formed from linoleic acid and glycerol is a liquid and not a...
- 14N.2.hl.TZ0.3a: A liquid boils when its vapour pressure equals atmospheric pressure. Determine the boiling...
- 14N.2.hl.TZ0.3b: Sketch another curve on the axes above to show how the vapour pressure of a liquid that has...
- 14N.2.hl.TZ0.10b: The oxygen in half-equation 2 is atmospheric oxygen that is found dissolved in water in very...
- 14N.1.sl.TZ0.12: Which process involves the breaking of hydrogen bonds? A. ...
- 14N.2.sl.TZ0.8b: The oxygen in half-equation 2 is atmospheric oxygen that is found dissolved in water in very...
- 13N.2.hl.TZ0.7c.ii: The interaction between an undissociated hydrogen halide molecule and a water molecule.
- 13N.2.hl.TZ0.8d: Explain why 2-methylbut-2-ene is less soluble in water than 2-methylbutan-2-ol.
- 13N.1.sl.TZ0.14: Which compound has the highest boiling point? A. ...
- 13N.2.sl.TZ0.6d: Explain why 2-methylbut-2-ene is less soluble in water than 2-methylbutan-2-ol.
- 13M.1.sl.TZ1.14: Which statements are correct about hydrogen bonding? I. It is an electrostatic...
- 13M.1.sl.TZ2.14: Which series shows increasing boiling points? A. ...
- 13M.2.sl.TZ2.4a: Identify the intermolecular forces present in hydrogen iodide in the liquid state, HI(l).
- 13M.2.sl.TZ2.4b.ii: State and explain which compound can form hydrogen bonds with water.
- 13M.2.sl.TZ2.4b.iii: Draw a diagram showing the resulting hydrogen bonds between water and the compound chosen in...
- 13M.2.sl.TZ2.6c.v: Suggest why monomers are often gases or volatile liquids whereas polymers are solids.
- 12N.2.sl.TZ0.4c: (i) Explain why metals are good conductors of electricity and why they are...
- 12N.2.sl.TZ0.1b: Although the molar masses of ICl and \({\rm{B}}{{\rm{r}}_2}\) are very similar, the boiling...
- 10N.2.hl.TZ0.2d: (i) Outline two reasons why the polymers of the alkenes are of economic importance. (ii)...
- 10N.2.hl.TZ0.7b: Hydrazine and ethene, C2H4, are hydrides of adjacent elements in the periodic table. The...
- 10N.1.sl.TZ0.13: Which order is correct when the following compounds are arranged in order of increasing...
- 10N.2.sl.TZ0.2c: (i) Outline two reasons why the polymers of the alkenes are of economic importance. (ii)...
- 10N.2.sl.TZ0.4c: The boiling point of hydrazine is much higher than that of ethene. Explain this difference in...
- 10N.2.sl.TZ0.5f: (i) Deduce the structural formula of each isomer. (ii) Identify the isomer from part...
- 10N.3.sl.TZ0.B2c: The formula of stearic acid is also given in Table 22 of the Data Booklet. Explain why...
- 09N.1.hl.TZ0.10: What is the correct order if the compounds are arranged in order of increasing boiling...
- 09N.1.sl.TZ0.13: Which compound does not form hydrogen bonds between its molecules? A. ...
- 09N.2.sl.TZ0.4a.i: Identify the boiling points for each of the isomers A, B and C and state a reason for your...
- 10M.1.sl.TZ2.13: Which substance can form intermolecular hydrogen bonds in the liquid state? A. ...
- 09M.2.sl.TZ1.7a.ii: Predict, with an explanation, which of the three compounds is least soluble or miscible in...
- 09M.2.sl.TZ1.1d: The reactants had to be stirred vigorously because they formed two distinct layers in the...
- 09M.2.sl.TZ1.7a.i: List the three compounds in order of increasing boiling point (lowest first) and explain the...
- 09M.3.sl.TZ1.B2c.iii: Determine whether cholesterol or lecithin is more soluble in water.
- 09M.1.sl.TZ2.10: Which statement best describes the intramolecular bonding in HCN(l)? A. Electrostatic...
- 09M.2.sl.TZ2.6a.v: Deduce and explain whether ethanol or A has the higher boiling point.
- 09M.2.sl.TZ2.7b.ii: The graph below shows the boiling points of the hydrides of group 5. Discuss the variation in...
- 11M.1.sl.TZ1.10: Which compound forms hydrogen bonds in the liquid state? A. ...
- 11M.2.sl.TZ1.7d.iii: Explain why silicon dioxide is a solid and carbon dioxide is a gas at room temperature.
- 11M.2.sl.TZ1.5b.ii: sodium oxide has a higher melting point than sulfur trioxide.
- 11M.2.sl.TZ1.6a: State and explain the trend of the boiling points of the first five members of the alkene...
- 11M.2.hl.TZ2.8b.iv: Explain whether the boiling point of 1-bromopentane will be higher, lower or the same as that...
- 11M.1.sl.TZ2.8: Which change explains why the boiling points of the halogens increase as their molecular...
- 11M.2.sl.TZ2.4: Methoxymethane, \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OC}}{{\text{H}}_{\text{3}}}\), and...
- 11M.2.sl.TZ2.5a.i: Draw the Lewis structure of ammonia and state the shape of the molecule and its bond angles.
- 11M.2.sl.TZ2.5a.ii: The conjugate acid of ammonia is the ammonium ion, \({\text{NH}}_4^ + \). Draw the Lewis...
- 12M.1.sl.TZ2.28: Which compound has the lowest boiling point? A. ...
- 12M.2.sl.TZ2.5b: (i) State and explain which of propan-1-ol,...
- 11N.2.hl.TZ0.9b.iv: Discuss the volatility of E compared to F.
- 11N.1.sl.TZ0.11: What is the correct order of increasing boiling points? A. ...
- 11N.2.sl.TZ0.4d: Based on the types of intermolecular force present, explain why butan-1-ol has a higher...
- 11N.2.sl.TZ0.7b.iv: Discuss the volatility of Y compared to Z.

