DP Chemistry Questionbank

Topic 8: Acids and bases
| Path: |
Description
[N/A]Directly related questions
- 20N.3.sl.TZ0.2g: Suggest a risk of using sulfuric acid as the catalyst.
-
17M.1.hl.TZ1.25:
What is the pH of 1.0 × 10−3 mol dm−3 sodium hydroxide, NaOH(aq)?
Kw = 1.0 × 10−14
A. 3
B. 4
C. 10
D. 11
-
17M.2.sl.TZ1.2f.ii:
Suggest one disadvantage of using this smoke in an enclosed space.
-
17M.2.sl.TZ2.7a.ii:
Identify one conjugate acid-base pair in the reaction.
-
17N.2.sl.TZ0.5c:
A student working in the laboratory classified HNO3, H2SO4, H3PO4 and HClO4 as acids based on their pH. He hypothesized that “all acids contain oxygen and hydrogen”.
Evaluate his hypothesis.
- 17N.2.sl.TZ0.5b.iii: State the conjugate base of the hydroxide ion, OH–.
-
18M.1.sl.TZ1.19:
Which classification is correct for the reaction?
H2PO4−(aq) + H2O(l) → HPO42−(aq) + H3O+(aq)
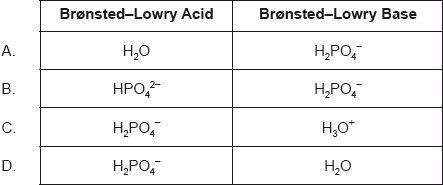
- 18M.1.sl.TZ2.20: Which statement is correct? A. A strong acid is a good proton donor and has a strong...
-
18M.2.sl.TZ2.2b.ii:
State and explain the effect on the rate of reaction if ethanoic acid of the same concentration is used in place of hydrochloric acid.
-
21N.2.sl.TZ0.5a:
Formulate an equation for the reaction of one mole of phosphoric acid with one mole of sodium hydroxide.
-
18N.2.sl.TZ0.6a:
State the equation for the reaction of each substance with water.
-
18N.1.sl.TZ0.19:
Which two species act as Brønsted–Lowry acids in the reaction?
H2PO4− (aq) + OH− (aq) HPO42− (aq) + H2O (l)
A. HPO42− (aq) and OH− (aq)
B. H2PO4− (aq) and HPO42− (aq)
C. HPO42− (aq) and H2O (l)
D. H2PO4− (aq) and H2O (l)
- 18N.3.hl.TZ0.1a: Outline why the initial reaction should be carried out under a fume hood.
-
18N.2.hl.TZ0.6a.i:
State the equation for the reaction of each substance with water.
- 22M.2.sl.TZ1.2e(i): State the relationship between NH4+ and NH3 in terms of the Brønsted–Lowry theory.
- 22M.2.sl.TZ2.3c(i): State the product formed from the reaction of SO3 with water.
-
19M.1.hl.TZ1.24:
Which solution is basic at 25 °C?
Kw = 1.0 × 10−14
A. [H+] = 1.0 × 10−3 mol dm−3
B. [OH−] = 1.0 × 10−13 mol dm−3
C. solution of pH = 4.00
D. [H3O+] = 1.0 × 10−13 mol dm−3
- 19M.1.sl.TZ1.20: Which is not a source of oxides of sulfur and nitrogen? A. burning coal B. internal combustion...
- 16N.2.sl.TZ0.2b: Suggest why it is more convenient to express acidity using the pH scale instead of using the...
-
20N.1.sl.TZ0.19:
Which substance will not produce copper(II) chloride when added to dilute hydrochloric acid?
A.
B.
C.
D.
-
20N.2.sl.TZ0.1c(ii):
State the formula of the conjugate base of hypochlorous acid.
- 20N.2.hl.TZ0.1c(i): Hypochlorous acid is considered a weak acid. Outline what is meant by the term weak acid.
- 17M.1.sl.TZ1.19: Which is an acid-base conjugate pair? A. H3O+ / OH– B. H2SO4 / SO42– C. CH3COOH /...
-
17M.3.sl.TZ1.2a:
Deduce why more heat was produced in mixture B than in mixture A.
-
17M.2.sl.TZ2.5c:
State the equation for the reaction of NO2 in the atmosphere to produce acid deposition.
-
17M.2.sl.TZ2.7b.iii:
Calculate [H+] in the NaOH solution.
-
17N.1.sl.TZ0.18:
What will happen if the pressure is increased in the following reaction mixture at equilibrium?
CO2 (g) + H2O (l) H+ (aq) + HCO3− (aq)
A. The equilibrium will shift to the right and pH will decrease.
B. The equilibrium will shift to the right and pH will increase.
C. The equilibrium will shift to the left and pH will increase.
D. The equilibrium will shift to the left and pH will decrease.
- 21M.2.sl.TZ1.2b(i): State the formula of its conjugate base.
-
21M.2.sl.TZ2.1f:
Outline how one calcium compound in the lime cycle can reduce a problem caused by acid deposition.
-
18M.2.hl.TZ2.2e.i:
Formulate the equation for the reaction of nitrogen dioxide, NO2, with water to form two acids.
-
18M.2.sl.TZ1.5a:
Predict, giving a reason, a difference between the reactions of the same concentrations of hydrochloric acid and ethanoic acid with samples of calcium carbonate.
-
18M.1.sl.TZ2.19:
Activity series of selected elements:
Which react with dilute sulfuric acid?
I. Cu
II. CuO
III. CuCO3
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
- 21N.1.hl.TZ0.27: What is correct for pure hot water?
- 21N.2.sl.TZ0.5d: Outline the reason that sodium hydroxide is considered a Brønsted–Lowry base.
- 21N.2.hl.TZ0.5d: Outline the reasons that sodium hydroxide is considered a Brønsted–Lowry and Lewis base.
-
22M.2.sl.TZ1.2e(iii):
Calculate the concentration of hydroxide ions in an ammonia solution with pH = 9.3. Use sections 1 and 2 of the data booklet.
-
22M.2.hl.TZ1.1d(iv):
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.
- 22M.2.sl.TZ2.3c(ii): State the meaning of a strong Brønsted–Lowry acid.
-
22M.2.hl.TZ2.6d(i):
State the product formed from the reaction of SO3 with water.
-
19M.2.hl.TZ2.5d(ii):
Predict, referring to Equilibrium (2), how the added sodium hydrogencarbonate affects the pH.(Assume pressure and temperature remain constant.)
-
19M.2.sl.TZ2.5b(i):
Predict, referring to Equilibrium (2), how the added sodium hydrogencarbonate affects the pH.(Assume pressure and temperature remain constant.)
-
19M.1.sl.TZ2.20:
What is the major reason why the pH of unpolluted rain is less than 7?
A. methane
B. carbon dioxide
C. nitrogen oxides
D. sulfur dioxide
- 20N.2.sl.TZ0.1c(i): Hypochlorous acid is considered a weak acid. Outline what is meant by the term weak acid.
-
17M.2.sl.TZ2.1c.i:
Some oxides of period 3, such as Na2O and P4O10, react with water. A spatula measure of each oxide was added to a separate 100 cm3 flask containing distilled water and a few drops of bromothymol blue indicator.
The indicator is listed in section 22 of the data booklet.
Deduce the colour of the resulting solution and the chemical formula of the product formed after reaction with water for each oxide.
-
21M.2.sl.TZ2.1d(iii):
Saturated calcium hydroxide solution is used to test for carbon dioxide. Calculate the pH of a 2.33 × 10−2 mol dm−3 solution of calcium hydroxide, a strong base.
-
21M.2.hl.TZ2.1c(iii):
Saturated calcium hydroxide solution is used to test for carbon dioxide. Calculate the pH of a 2.33 × 10−2 mol dm−3 solution of calcium hydroxide, a strong base.
-
21M.2.hl.TZ2.1e:
Outline how one calcium compound in the lime cycle can reduce a problem caused by acid deposition.
-
18M.2.hl.TZ1.5a:
Predict, giving a reason, a difference between the reactions of the same concentrations of hydrochloric acid and ethanoic acid with samples of calcium carbonate.
-
21N.2.hl.TZ0.5a:
Formulate an equation for the reaction of one mole of phosphoric acid with one mole of sodium hydroxide.
- 22M.1.sl.TZ1.20: Which 0.01 mol dm–3 aqueous solution has the highest pH? A. HCl B. H2SO4 C. NaOH D. NH3
-
22M.1.sl.TZ2.30:
20 cm3 of 1 mol dm−3 sulfuric acid was added dropwise to 20 cm3 of 1 mol dm−3 barium hydroxide producing a precipitate of barium sulfate.
H2SO4 (aq) + Ba(OH)2 (aq) → 2H2O (l) + BaSO4 (s)
Which graph represents a plot of conductivity against volume of acid added?
- 22M.2.hl.TZ2.6d(ii): State the meaning of a strong Brønsted–Lowry acid.
-
19M.2.hl.TZ1.2f(i):
The pH of an aqueous solution of benzoic acid at 298 K is 2.95. Determine the concentration of hydroxide ions in the solution, using section 2 of the data booklet.
-
19M.2.hl.TZ2.5a(i):
Distinguish between a weak and strong acid.
Weak acid:
Strong acid:
-
19M.1.hl.TZ2.25:
What is the major reason why the pH of unpolluted rain is less than 7?
A. methane
B. carbon dioxide
C. nitrogen oxides
D. sulfur dioxide
-
19M.1.sl.TZ2.19:
What is the pH of 0.001 mol dm−3 NaOH (aq)?
A. 1
B. 3
C. 11
D. 13
- 19N.2.hl.TZ0.4b: Outline two laboratory methods of distinguishing between solutions of citric acid and...
-
19N.3.sl.TZ0.16d:
Calculate the pH of a buffer solution which contains 0.20 mol dm−3 ethanoic acid and 0.50 mol dm−3 sodium ethanoate. Use section 1 of the data booklet.
pKa (ethanoic acid) = 4.76
-
19N.3.sl.TZ0.16b:
An antacid contains calcium carbonate and magnesium carbonate.
Write the equation for the reaction of magnesium carbonate with excess stomach acid.
- 19N.2.sl.TZ0.4b: Outline one laboratory methods of distinguishing between solutions of citric acid and...
- 16N.1.sl.TZ0.27: A student carried out a titration to determine the concentration of an acid and found that his...
-
16N.2.hl.TZ0.2c:
5.00 g of an impure sample of hydrated ethanedioic acid, (COOH)2•2H2O, was dissolved in water to make 1.00 dm3 of solution. 25.0 cm3 samples of this solution were titrated against a 0.100 mol dm-3 solution of sodium hydroxide using a suitable indicator.
(COOH)2 (aq) + 2NaOH (aq) → (COONa)2 (aq) + 2H2O (l)
The mean value of the titre was 14.0 cm3.
(i) Suggest a suitable indicator for this titration. Use section 22 of the data booklet.
(ii) Calculate the amount, in mol, of NaOH in 14.0 cm3 of 0.100 mol dm-3 solution.
(iii) Calculate the amount, in mol, of ethanedioic acid in each 25.0 cm3 sample.
(iv) Determine the percentage purity of the hydrated ethanedioic acid sample.
-
20N.1.sl.TZ0.16:
Which apparatus can be used to monitor the rate of this reaction?
- A pH meter
- A gas syringe
- A colorimeter
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
-
20N.1.hl.TZ0.27:
What is the pH of an ammonia solution that has ?
A.
B.
C.
D.
-
20N.1.hl.TZ0.24:
Which of these oxides contribute to acid deposition?
I.
II.
III.A. I and II only
B. I and III only
C. II and III only
D. I, II and III
-
20N.1.hl.TZ0.26:
Which species is a Lewis acid but not a Brønsted–Lowry acid?
A.
B.
C.
D.
- 20N.2.hl.TZ0.5f(i): Potassium hydroxide solutions can react with carbon dioxide from the air. The solution was made...
-
17M.2.sl.TZ1.4c:
Hydrazine reacts with water in a similar way to ammonia. Deduce an equation for the reaction of hydrazine with water.
-
17M.3.sl.TZ1.2b:
Deduce why the temperature is higher in mixture C than in mixture D.
-
17M.2.sl.TZ2.7b.i:
Calculate the amount, in mol, of NaOH(aq) used.
-
17M.3.hl.TZ2.4a:
Identify the other product formed.
-
21M.2.sl.TZ1.2b(ii):
Saturated aqueous hydrogen sulfide has a concentration of 0.10 mol dm−3 and a pH of 4.0. Demonstrate whether it is a strong or weak acid.
-
18M.2.hl.TZ2.2d.iii:
Outline, using an equation, why sodium ethanoate is basic.
- 21N.1.sl.TZ0.21: What is the conjugate acid of HS−? A. H2S B. S2− C. H2SO3 D. H2SO4
-
21N.2.sl.TZ0.5b:
Formulate two equations to show the amphiprotic nature of H2PO4−.
-
18N.1.sl.TZ0.20:
What is the order of increasing pH for the following solutions of the same concentration?
A. HCl (aq) < NH3 (aq) < NaOH (aq) < CH3COOH (aq)
B. CH3COOH (aq) < HCl (aq) < NH3 (aq) < NaOH (aq)
C. HCl (aq) < CH3COOH (aq) < NH3 (aq) < NaOH (aq)
D. NaOH (aq) < NH3 (aq) < CH3COOH (aq) < HCl (aq)
-
22M.1.hl.TZ2.24:
What happens to the amount of hydroxide ions and hydroxide ion concentration when water is added to a solution of NH3 (aq)?
-
22M.2.hl.TZ1.4c(i):
Calculate the concentration of hydroxide ions in an ammonia solution with pH = 9.3. Use sections 1 and 2 of the data booklet.
-
19M.2.sl.TZ2.5a(i):
Distinguish between a weak and strong acid.
Weak acid:
Strong acid:
-
19M.1.sl.TZ1.19:
Which solution is basic at 25 °C?
Kw = 1.0 × 10−14
A. [H+] = 1.0 × 10−3 mol dm−3
B. [OH−] = 1.0 × 10−13 mol dm−3
C. solution of pH = 4.00
D. [H3O+] = 1.0 × 10−13 mol dm−3
- 19N.2.hl.TZ0.4a(i): Identify a conjugate acid–base pair in the equation.
-
19N.3.sl.TZ0.8b:
Explain why a change in pH affects the tertiary structure of an enzyme in solution.
-
16N.2.sl.TZ0.2c:
5.00 g of an impure sample of hydrated ethanedioic acid, (COOH)2•2H2O, was dissolved in water to make 1.00 dm3 of solution. 25.0 cm3 samples of this solution were titrated against a 0.100 mol dm-3 solution of sodium hydroxide using a suitable indicator.
(COOH)2 (aq) + 2NaOH (aq) → (COONa)2 (aq) + 2H2O (l)
The mean value of the titre was 14.0 cm3.
(i) Calculate the amount, in mol, of NaOH in 14.0 cm3 of 0.100 mol dm-3 solution.
(ii) Calculate the amount, in mol, of ethanedioic acid in each 25.0 cm3 sample.
(iii) Determine the percentage purity of the hydrated ethanedioic acid sample.
-
20N.2.hl.TZ0.1c(ii):
State the formula of the conjugate base of hypochlorous acid.
-
20N.2.hl.TZ0.5d:
Calculate the of the conjugate base of ethanoic acid using sections 2 and 21 of the data booklet.
-
20N.3.sl.TZ0.2d:
An additional experiment was conducted in which only the sulfuric acid catalyst was titrated with . Outline why this experiment was necessary.
-
17M.1.hl.TZ1.26:
Which species acts as a Lewis and Brønsted–Lowry base?
A. [Al(H2O)6]3+
B. BF3
C. NH4+
D. OH−
-
17M.2.sl.TZ2.7a.i:
Identify the amphiprotic species.
-
17M.3.sl.TZ2.14b.i:
Oceans can act as a carbon sink, removing some CO2(g) from the atmosphere.
CO2(g) CO2(aq)
Aqueous carbon dioxide, CO2(aq), quickly reacts with ocean water in a new equilibrium reaction. Construct the equilibrium equation for this reaction including state symbols.
- 17N.2.hl.TZ0.1e: Suggest why the enthalpy change of neutralization of CH3COOH is less negative than that of HCl.
- 17N.2.sl.TZ0.5b.ii: State what is meant by the term conjugate base.
-
21M.1.hl.TZ1.26:
Which is a Lewis acid, but not a Brønsted-Lowry acid?
A.
B.
C.
D.
- 21M.1.sl.TZ2.19: Which cannot act as a Brønsted–Lowry base? A. HPO42− B. H2O C. CH4 D. NH3
- 21M.2.sl.TZ1.1d(iii): Suggest why this process might raise environmental concerns.
-
18M.2.sl.TZ2.2c:
Outline why pH is more widely used than [H+] for measuring relative acidity.
- 21N.1.hl.TZ0.26: What is a possible value of pH at the equivalence point in the titration of a strong acid with a...
-
21N.2.hl.TZ0.5b:
Formulate two equations to show the amphiprotic nature of H2PO4−.
-
18N.2.hl.TZ0.6b.i:
A 0.250 mol dm−3 aqueous solution of butanoic acid has a concentration of hydrogen ions, [H+], of 0.00192 mol dm−3. Calculate the concentration of hydroxide ions, [OH−], in the solution at 298 K.
-
22M.2.sl.TZ1.1d(iii):
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.
-
19M.2.hl.TZ2.5a(ii):
The hydrogencarbonate ion, produced in Equilibrium (2), can also act as an acid.
State the formula of its conjugate base.
-
19M.1.hl.TZ1.25:
With which do most acids react?
I. sodium hydrogen carbonate
II. magnesium
III. calcium sulfateA. I and II only
B. I and III only
C. II and III only
D. I, II and III
- 19N.3.sl.TZ0.1c(i): Justify this hypothesis.
- 19N.2.sl.TZ0.4a(ii): The value of the equilibrium constant for the first dissociation at 298 K is 5.01 × 10−4. State,...
- 16N.1.sl.TZ0.20: What occurs when solid sodium hydrogen carbonate reacts with aqueous sulfuric acid? A. Bubbles...
-
20N.2.hl.TZ0.1c(iii):
Calculate the concentration of in a solution with a .
-
17M.2.hl.TZ2.8b.v:
State a technique other than a pH titration that can be used to detect the equivalence point.
-
17N.3.sl.TZ0.2a:
State an equation for the reaction of magnesium hydroxide with hydrochloric acid.
-
21M.1.sl.TZ1.20:
Which solution has a pH of 9?
A. 1.0 × 10−9 mol dm−3 (aq)
B. 1.0 × 10−5 mol dm−3 (aq)
C. 1.0 × 10−9 mol dm−3 (aq)
D. 1.0 × 10−5 mol dm−3 (aq)
- 18M.1.sl.TZ1.20: What are the products of the reaction between sulfuric acid and sodium hydrogen carbonate? A. ...
-
18M.2.sl.TZ2.2d:
Outline why H3PO4/HPO42− is not a conjugate acid-base pair.
-
19M.2.hl.TZ2.5e:
The reaction of the hydroxide ion with carbon dioxide and with the hydrogencarbonate ion can be represented by Equations 3 and 4.
Equation (3) OH− (aq) + CO2 (g) → HCO3− (aq)
Equation (4) OH− (aq) + HCO3− (aq) → H2O (l) + CO32− (aq)Discuss how these equations show the difference between a Lewis base and a Brønsted–Lowry base.
Equation (3):
Equation (4):
-
19M.2.sl.TZ1.2b(i):
The pH of an aqueous solution of benzoic acid at 298 K is 2.95. Determine the concentration of hydroxide ions in the solution, using section 2 of the data booklet.
- 19N.1.sl.TZ0.20: What is the difference between a conjugate Brønsted–Lowry acid–base pair? A. Electron pair B. ...
- 19N.1.sl.TZ0.21: Which is an example of an amphiprotic species? A. Al2O3 B. CO32− C. P4O10 D. HPO42−
- 16N.1.sl.TZ0.19: Which species behave as Brønsted–Lowry bases in the following reaction? H2SO4 + HNO3 H2NO3+ +...
- 16N.2.sl.TZ0.2a: Distinguish between a weak acid and a strong acid. Weak acid: Strong acid:
-
20N.1.sl.TZ0.20:
Which of these acids has the weakest conjugate base?
A.
B.
C.
D.
-
17M.2.sl.TZ1.4d:
Outline, using an ionic equation, what is observed when magnesium powder is added to a solution of ammonium chloride.
-
17M.3.sl.TZ2.14b.ii:
Describe how large amounts of CO2 could reduce the pH of the ocean using an equation to support your answer.
- 17N.2.sl.TZ0.5b.i: Identify two different amphiprotic species in the above reactions.
- 17N.1.sl.TZ0.19: 10.0 cm3 of an aqueous solution of sodium hydroxide of pH = 10 is mixed with 990.0 cm3 of...
- 21M.1.sl.TZ2.20: Which causes acid deposition? A. SO2 B. SiO2 C. SrO D. CO2
- 21M.2.sl.TZ1.2b(iii): Calculate the hydroxide ion concentration in saturated aqueous hydrogen sulfide.
-
18M.2.hl.TZ2.2e.ii:
Formulate the equation for the reaction of one of the acids produced in (e)(i) with calcium carbonate.
-
18M.2.sl.TZ1.5b:
Dissolved carbon dioxide causes unpolluted rain to have a pH of approximately 5, but other dissolved gases can result in a much lower pH. State one environmental effect of acid rain.
- 18N.3.sl.TZ0.1a: Outline why the initial reaction should be carried out under a fume hood.
-
22M.1.sl.TZ2.19:
Which of the 0.001 mol dm−3 solutions is most likely to have a pH of 11.3?
A. Ca(OH)2 (aq)
B. H3PO4 (aq)
C. NaOH (aq)
D. NH4OH (aq)
-
22M.1.sl.TZ2.20:
What is the strongest acid in the equation below?
H3AsO4 + H2O H2AsO4− + H3O+ Kc = 4.5 × 10−4
A. H3AsO4
B. H2O
C. H2AsO4−
D. H3O+
- 22M.2.hl.TZ1.4a: State the relationship between NH4+ and NH3 in terms of the Brønsted–Lowry theory.
-
19M.1.hl.TZ2.24:
What is the pH of 0.001 mol dm−3 NaOH (aq)?
A. 1
B. 3
C. 11
D. 13
-
19M.2.sl.TZ1.2a:
Draw the structure of the conjugate base of benzoic acid showing all the atoms and all the bonds.
-
19N.3.sl.TZ0.16a:
Identify the compound responsible for the acidity of gastric juice, and state whether it is a strong or weak acid.
- 19N.2.sl.TZ0.4a(i): Identify a conjugate acid–base pair in the equation.
-
19N.2.sl.TZ0.4a(iii):
The dissociation of citric acid is an endothermic process. State the effect on the hydrogen ion concentration, [H+], and on the equilibrium constant, of increasing the temperature.
-
16N.2.sl.TZ0.3b:
Suggest why the experiment should be carried out in a fume hood or in a well-ventilated laboratory.
-
20N.2.sl.TZ0.1c(iii):
Calculate the concentration of in a solution with a .
-
20N.2.hl.TZ0.5c:
State the expression for ethanoic acid.
-
20N.2.hl.TZ0.5f(ii):
Potassium hydroxide solutions can react with carbon dioxide from the air. The solution was made one day prior to using it in the titration.
Predict, giving a reason, the effect of this error on the calculated concentration of ethanoic acid in 5(e).
-
17M.1.sl.TZ1.20:
Which 1.0 moldm–3 solution has the highest pH?
A. Ammonium chloride
B. Sulfuric acid
C. Sodium chloride
D. Ammonia
- 17M.1.hl.TZ1.24: Which species produced by the successive dissociations of phosphoric acid, H3PO4,...
- 17M.1.sl.TZ2.20: Which of the following is correct? A. A weak acid is a proton donor and its aqueous solution...
-
17N.1.sl.TZ0.20:
Which statement is incorrect for a 0.10 mol dm–3 HCOOH solution?
A. pH = 1
B. [H+] << 0.10 mol dm–3
C. [HCOO–] is approximately equal to [H+]
D. HCOOH is partially ionized
- 21M.2.hl.TZ1.1e(iii): Suggest why this process might raise environmental concerns.
- 18M.1.hl.TZ1.25: What is the pH of a solution in which the hydroxide ion concentration is 1 × 10−11 mol dm−3 at...
-
18M.2.hl.TZ1.5b:
Dissolved carbon dioxide causes unpolluted rain to have a pH of approximately 5, but other dissolved gases can result in a much lower pH. State one environmental effect of acid rain.
- 18M.1.hl.TZ1.24: What describes HPO42−? A. Amphiprotic but not amphoteric B. Amphoteric but not...
-
18M.2.hl.TZ1.5c:
Write an equation to show ammonia, NH3, acting as a Brønsted–Lowry base and a different equation to show it acting as a Lewis base.
- 21N.1.sl.TZ0.20: Which ions are present in an aqueous solution of Na2CO3? I. HCO3−II. OH−III. CO32− A. I and...
-
21N.1.hl.TZ0.25:
What is the pH of 0.01 mol dm−3 KOH (aq)?
A. 1.0B. 2.0
C. 12.0
D. 13.0
- 18N.2.sl.TZ0.6c: State the formula of the salt formed when butanoic acid reacts with ethylamine.
-
19M.2.hl.TZ1.2d:
Draw the structure of the conjugate base of benzoic acid showing all the atoms and all the bonds.
-
19M.2.hl.TZ1.5a:
Outline why ethanoic acid is classified as a weak acid.
-
19M.2.sl.TZ1.5a:
Outline why ethanoic acid is classified as a weak acid.
-
19M.2.sl.TZ2.5a(ii):
The hydrogencarbonate ion, produced in Equilibrium (2), can also act as an acid.
State the formula of its conjugate base.
-
19N.2.hl.TZ0.4a(iii):
The dissociation of citric acid is an endothermic process. State the effect on the hydrogen ion concentration, [H+], and on Ka, of increasing the temperature.
- 19N.2.hl.TZ0.4a(ii): The value of Ka at 298 K for the first dissociation is 5.01 × 10−4. State, giving a reason, the...
Sub sections and their related questions
8.1 Theories of acids and bases
- 16N.1.sl.TZ0.19: Which species behave as Brønsted–Lowry bases in the following reaction? H2SO4 + HNO3 H2NO3+ +...
- 17M.1.sl.TZ1.19: Which is an acid-base conjugate pair? A. H3O+ / OH– B. H2SO4 / SO42– C. CH3COOH /...
- 17M.1.hl.TZ1.24: Which species produced by the successive dissociations of phosphoric acid, H3PO4,...
-
17M.1.hl.TZ1.26:
Which species acts as a Lewis and Brønsted–Lowry base?
A. [Al(H2O)6]3+
B. BF3
C. NH4+
D. OH−
- 17M.1.sl.TZ2.20: Which of the following is correct? A. A weak acid is a proton donor and its aqueous solution...
-
17M.2.sl.TZ2.7a.i:
Identify the amphiprotic species.
-
17M.2.sl.TZ2.7a.ii:
Identify one conjugate acid-base pair in the reaction.
-
17M.2.sl.TZ2.7b.i:
Calculate the amount, in mol, of NaOH(aq) used.
- 17N.2.sl.TZ0.5b.i: Identify two different amphiprotic species in the above reactions.
- 17N.2.sl.TZ0.5b.ii: State what is meant by the term conjugate base.
- 17N.2.sl.TZ0.5b.iii: State the conjugate base of the hydroxide ion, OH–.
-
17N.2.sl.TZ0.5c:
A student working in the laboratory classified HNO3, H2SO4, H3PO4 and HClO4 as acids based on their pH. He hypothesized that “all acids contain oxygen and hydrogen”.
Evaluate his hypothesis.
- 18M.1.hl.TZ1.24: What describes HPO42−? A. Amphiprotic but not amphoteric B. Amphoteric but not...
-
18M.2.hl.TZ1.5c:
Write an equation to show ammonia, NH3, acting as a Brønsted–Lowry base and a different equation to show it acting as a Lewis base.
-
18M.1.sl.TZ1.19:
Which classification is correct for the reaction?
H2PO4−(aq) + H2O(l) → HPO42−(aq) + H3O+(aq)

-
18M.2.sl.TZ2.2d:
Outline why H3PO4/HPO42− is not a conjugate acid-base pair.
-
18N.1.sl.TZ0.19:
Which two species act as Brønsted–Lowry acids in the reaction?
H2PO4− (aq) + OH− (aq) HPO42− (aq) + H2O (l)
A. HPO42− (aq) and OH− (aq)
B. H2PO4− (aq) and HPO42− (aq)
C. HPO42− (aq) and H2O (l)
D. H2PO4− (aq) and H2O (l)
-
18N.2.sl.TZ0.6a:
State the equation for the reaction of each substance with water.
-
18N.2.hl.TZ0.6a.i:
State the equation for the reaction of each substance with water.
-
19M.2.hl.TZ1.2d:
Draw the structure of the conjugate base of benzoic acid showing all the atoms and all the bonds.
-
19M.2.hl.TZ2.5a(ii):
The hydrogencarbonate ion, produced in Equilibrium (2), can also act as an acid.
State the formula of its conjugate base.
-
19M.2.hl.TZ2.5e:
The reaction of the hydroxide ion with carbon dioxide and with the hydrogencarbonate ion can be represented by Equations 3 and 4.
Equation (3) OH− (aq) + CO2 (g) → HCO3− (aq)
Equation (4) OH− (aq) + HCO3− (aq) → H2O (l) + CO32− (aq)Discuss how these equations show the difference between a Lewis base and a Brønsted–Lowry base.
Equation (3):
Equation (4):
-
19M.2.sl.TZ1.2a:
Draw the structure of the conjugate base of benzoic acid showing all the atoms and all the bonds.
-
19M.2.sl.TZ2.5a(ii):
The hydrogencarbonate ion, produced in Equilibrium (2), can also act as an acid.
State the formula of its conjugate base.
- 19N.2.hl.TZ0.4a(i): Identify a conjugate acid–base pair in the equation.
- 19N.3.sl.TZ0.1c(i): Justify this hypothesis.
- 19N.1.sl.TZ0.20: What is the difference between a conjugate Brønsted–Lowry acid–base pair? A. Electron pair B. ...
- 19N.1.sl.TZ0.21: Which is an example of an amphiprotic species? A. Al2O3 B. CO32− C. P4O10 D. HPO42−
- 19N.2.sl.TZ0.4a(i): Identify a conjugate acid–base pair in the equation.
-
20N.1.sl.TZ0.20:
Which of these acids has the weakest conjugate base?
A.
B.
C.
D.
-
20N.1.hl.TZ0.26:
Which species is a Lewis acid but not a Brønsted–Lowry acid?
A.
B.
C.
D.
-
20N.2.sl.TZ0.1c(ii):
State the formula of the conjugate base of hypochlorous acid.
-
20N.2.hl.TZ0.1c(ii):
State the formula of the conjugate base of hypochlorous acid.
-
20N.2.hl.TZ0.5d:
Calculate the of the conjugate base of ethanoic acid using sections 2 and 21 of the data booklet.
-
21M.1.hl.TZ1.26:
Which is a Lewis acid, but not a Brønsted-Lowry acid?
A.
B.
C.
D.
- 21M.1.sl.TZ2.19: Which cannot act as a Brønsted–Lowry base? A. HPO42− B. H2O C. CH4 D. NH3
- 21M.2.sl.TZ1.2b(i): State the formula of its conjugate base.
- 21N.1.sl.TZ0.21: What is the conjugate acid of HS−? A. H2S B. S2− C. H2SO3 D. H2SO4
-
21N.2.sl.TZ0.5a:
Formulate an equation for the reaction of one mole of phosphoric acid with one mole of sodium hydroxide.
-
21N.2.sl.TZ0.5b:
Formulate two equations to show the amphiprotic nature of H2PO4−.
- 21N.2.sl.TZ0.5d: Outline the reason that sodium hydroxide is considered a Brønsted–Lowry base.
-
21N.2.hl.TZ0.5a:
Formulate an equation for the reaction of one mole of phosphoric acid with one mole of sodium hydroxide.
-
21N.2.hl.TZ0.5b:
Formulate two equations to show the amphiprotic nature of H2PO4−.
- 21N.2.hl.TZ0.5d: Outline the reasons that sodium hydroxide is considered a Brønsted–Lowry and Lewis base.
- 22M.2.sl.TZ1.2e(i): State the relationship between NH4+ and NH3 in terms of the Brønsted–Lowry theory.
- 22M.2.hl.TZ1.4a: State the relationship between NH4+ and NH3 in terms of the Brønsted–Lowry theory.
- 22M.2.sl.TZ2.3c(ii): State the meaning of a strong Brønsted–Lowry acid.
- 22M.2.hl.TZ2.6d(ii): State the meaning of a strong Brønsted–Lowry acid.
8.2 Properties of acids and bases
- 16N.1.sl.TZ0.20: What occurs when solid sodium hydrogen carbonate reacts with aqueous sulfuric acid? A. Bubbles...
- 16N.1.sl.TZ0.27: A student carried out a titration to determine the concentration of an acid and found that his...
-
16N.2.sl.TZ0.2c:
5.00 g of an impure sample of hydrated ethanedioic acid, (COOH)2•2H2O, was dissolved in water to make 1.00 dm3 of solution. 25.0 cm3 samples of this solution were titrated against a 0.100 mol dm-3 solution of sodium hydroxide using a suitable indicator.
(COOH)2 (aq) + 2NaOH (aq) → (COONa)2 (aq) + 2H2O (l)
The mean value of the titre was 14.0 cm3.
(i) Calculate the amount, in mol, of NaOH in 14.0 cm3 of 0.100 mol dm-3 solution.
(ii) Calculate the amount, in mol, of ethanedioic acid in each 25.0 cm3 sample.
(iii) Determine the percentage purity of the hydrated ethanedioic acid sample.
-
16N.2.hl.TZ0.2c:
5.00 g of an impure sample of hydrated ethanedioic acid, (COOH)2•2H2O, was dissolved in water to make 1.00 dm3 of solution. 25.0 cm3 samples of this solution were titrated against a 0.100 mol dm-3 solution of sodium hydroxide using a suitable indicator.
(COOH)2 (aq) + 2NaOH (aq) → (COONa)2 (aq) + 2H2O (l)
The mean value of the titre was 14.0 cm3.
(i) Suggest a suitable indicator for this titration. Use section 22 of the data booklet.
(ii) Calculate the amount, in mol, of NaOH in 14.0 cm3 of 0.100 mol dm-3 solution.
(iii) Calculate the amount, in mol, of ethanedioic acid in each 25.0 cm3 sample.
(iv) Determine the percentage purity of the hydrated ethanedioic acid sample.
-
17M.2.sl.TZ1.2f.ii:
Suggest one disadvantage of using this smoke in an enclosed space.
-
17M.3.sl.TZ1.2a:
Deduce why more heat was produced in mixture B than in mixture A.
-
17M.3.sl.TZ1.2b:
Deduce why the temperature is higher in mixture C than in mixture D.
-
17M.2.sl.TZ2.1c.i:
Some oxides of period 3, such as Na2O and P4O10, react with water. A spatula measure of each oxide was added to a separate 100 cm3 flask containing distilled water and a few drops of bromothymol blue indicator.
The indicator is listed in section 22 of the data booklet.
Deduce the colour of the resulting solution and the chemical formula of the product formed after reaction with water for each oxide.
-
17M.2.hl.TZ2.8b.v:
State a technique other than a pH titration that can be used to detect the equivalence point.
-
17N.3.sl.TZ0.2a:
State an equation for the reaction of magnesium hydroxide with hydrochloric acid.
-
18M.2.hl.TZ2.2d.iii:
Outline, using an equation, why sodium ethanoate is basic.
- 18M.1.sl.TZ1.20: What are the products of the reaction between sulfuric acid and sodium hydrogen carbonate? A. ...
-
18M.1.sl.TZ2.19:
Activity series of selected elements:
Which react with dilute sulfuric acid?
I. Cu
II. CuO
III. CuCO3
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
- 18N.2.sl.TZ0.6c: State the formula of the salt formed when butanoic acid reacts with ethylamine.
-
19M.1.hl.TZ1.25:
With which do most acids react?
I. sodium hydrogen carbonate
II. magnesium
III. calcium sulfateA. I and II only
B. I and III only
C. II and III only
D. I, II and III
-
19N.3.sl.TZ0.16b:
An antacid contains calcium carbonate and magnesium carbonate.
Write the equation for the reaction of magnesium carbonate with excess stomach acid.
-
20N.1.sl.TZ0.19:
Which substance will not produce copper(II) chloride when added to dilute hydrochloric acid?
A.
B.
C.
D.
-
20N.2.hl.TZ0.5c:
State the expression for ethanoic acid.
- 20N.2.hl.TZ0.5f(i): Potassium hydroxide solutions can react with carbon dioxide from the air. The solution was made...
-
20N.2.hl.TZ0.5f(ii):
Potassium hydroxide solutions can react with carbon dioxide from the air. The solution was made one day prior to using it in the titration.
Predict, giving a reason, the effect of this error on the calculated concentration of ethanoic acid in 5(e).
-
20N.3.sl.TZ0.2d:
An additional experiment was conducted in which only the sulfuric acid catalyst was titrated with . Outline why this experiment was necessary.
- 20N.3.sl.TZ0.2g: Suggest a risk of using sulfuric acid as the catalyst.
- 21N.1.hl.TZ0.26: What is a possible value of pH at the equivalence point in the titration of a strong acid with a...
-
22M.1.hl.TZ2.24:
What happens to the amount of hydroxide ions and hydroxide ion concentration when water is added to a solution of NH3 (aq)?
-
22M.2.sl.TZ1.1d(iii):
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.
-
22M.2.hl.TZ1.1d(iv):
Deduce, giving reasons, whether the reaction of magnesium nitride with water is an acid–base reaction, a redox reaction, neither or both.
8.3 The pH scale
- 16N.2.sl.TZ0.2b: Suggest why it is more convenient to express acidity using the pH scale instead of using the...
-
17M.1.hl.TZ1.25:
What is the pH of 1.0 × 10−3 mol dm−3 sodium hydroxide, NaOH(aq)?
Kw = 1.0 × 10−14
A. 3
B. 4
C. 10
D. 11
-
17M.2.sl.TZ2.7b.iii:
Calculate [H+] in the NaOH solution.
-
17N.1.sl.TZ0.18:
What will happen if the pressure is increased in the following reaction mixture at equilibrium?
CO2 (g) + H2O (l) H+ (aq) + HCO3− (aq)
A. The equilibrium will shift to the right and pH will decrease.
B. The equilibrium will shift to the right and pH will increase.
C. The equilibrium will shift to the left and pH will increase.
D. The equilibrium will shift to the left and pH will decrease.
- 17N.1.sl.TZ0.19: 10.0 cm3 of an aqueous solution of sodium hydroxide of pH = 10 is mixed with 990.0 cm3 of...
-
17N.1.sl.TZ0.20:
Which statement is incorrect for a 0.10 mol dm–3 HCOOH solution?
A. pH = 1
B. [H+] << 0.10 mol dm–3
C. [HCOO–] is approximately equal to [H+]
D. HCOOH is partially ionized
- 18M.1.hl.TZ1.25: What is the pH of a solution in which the hydroxide ion concentration is 1 × 10−11 mol dm−3 at...
-
18M.2.sl.TZ2.2c:
Outline why pH is more widely used than [H+] for measuring relative acidity.
-
18N.2.hl.TZ0.6b.i:
A 0.250 mol dm−3 aqueous solution of butanoic acid has a concentration of hydrogen ions, [H+], of 0.00192 mol dm−3. Calculate the concentration of hydroxide ions, [OH−], in the solution at 298 K.
-
19M.2.hl.TZ1.2f(i):
The pH of an aqueous solution of benzoic acid at 298 K is 2.95. Determine the concentration of hydroxide ions in the solution, using section 2 of the data booklet.
-
19M.2.hl.TZ2.5d(ii):
Predict, referring to Equilibrium (2), how the added sodium hydrogencarbonate affects the pH.(Assume pressure and temperature remain constant.)
-
19M.1.hl.TZ1.24:
Which solution is basic at 25 °C?
Kw = 1.0 × 10−14
A. [H+] = 1.0 × 10−3 mol dm−3
B. [OH−] = 1.0 × 10−13 mol dm−3
C. solution of pH = 4.00
D. [H3O+] = 1.0 × 10−13 mol dm−3
-
19M.1.hl.TZ2.24:
What is the pH of 0.001 mol dm−3 NaOH (aq)?
A. 1
B. 3
C. 11
D. 13
-
19M.2.sl.TZ1.2b(i):
The pH of an aqueous solution of benzoic acid at 298 K is 2.95. Determine the concentration of hydroxide ions in the solution, using section 2 of the data booklet.
-
19M.2.sl.TZ2.5b(i):
Predict, referring to Equilibrium (2), how the added sodium hydrogencarbonate affects the pH.(Assume pressure and temperature remain constant.)
-
19M.1.sl.TZ1.19:
Which solution is basic at 25 °C?
Kw = 1.0 × 10−14
A. [H+] = 1.0 × 10−3 mol dm−3
B. [OH−] = 1.0 × 10−13 mol dm−3
C. solution of pH = 4.00
D. [H3O+] = 1.0 × 10−13 mol dm−3
-
19M.1.sl.TZ2.19:
What is the pH of 0.001 mol dm−3 NaOH (aq)?
A. 1
B. 3
C. 11
D. 13
-
19N.3.sl.TZ0.16d:
Calculate the pH of a buffer solution which contains 0.20 mol dm−3 ethanoic acid and 0.50 mol dm−3 sodium ethanoate. Use section 1 of the data booklet.
pKa (ethanoic acid) = 4.76
-
19N.3.sl.TZ0.8b:
Explain why a change in pH affects the tertiary structure of an enzyme in solution.
-
20N.1.sl.TZ0.16:
Which apparatus can be used to monitor the rate of this reaction?
- A pH meter
- A gas syringe
- A colorimeter
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
-
20N.1.hl.TZ0.27:
What is the pH of an ammonia solution that has ?
A.
B.
C.
D.
-
20N.2.sl.TZ0.1c(iii):
Calculate the concentration of in a solution with a .
-
20N.2.hl.TZ0.1c(iii):
Calculate the concentration of in a solution with a .
-
21M.1.sl.TZ1.20:
Which solution has a pH of 9?
A. 1.0 × 10−9 mol dm−3 (aq)
B. 1.0 × 10−5 mol dm−3 (aq)
C. 1.0 × 10−9 mol dm−3 (aq)
D. 1.0 × 10−5 mol dm−3 (aq)
- 21M.2.sl.TZ1.2b(iii): Calculate the hydroxide ion concentration in saturated aqueous hydrogen sulfide.
-
21M.2.sl.TZ2.1d(iii):
Saturated calcium hydroxide solution is used to test for carbon dioxide. Calculate the pH of a 2.33 × 10−2 mol dm−3 solution of calcium hydroxide, a strong base.
-
21M.2.hl.TZ2.1c(iii):
Saturated calcium hydroxide solution is used to test for carbon dioxide. Calculate the pH of a 2.33 × 10−2 mol dm−3 solution of calcium hydroxide, a strong base.
-
21N.1.hl.TZ0.25:
What is the pH of 0.01 mol dm−3 KOH (aq)?
A. 1.0B. 2.0
C. 12.0
D. 13.0
- 21N.1.hl.TZ0.27: What is correct for pure hot water?
- 22M.1.sl.TZ1.20: Which 0.01 mol dm–3 aqueous solution has the highest pH? A. HCl B. H2SO4 C. NaOH D. NH3
-
22M.1.sl.TZ2.19:
Which of the 0.001 mol dm−3 solutions is most likely to have a pH of 11.3?
A. Ca(OH)2 (aq)
B. H3PO4 (aq)
C. NaOH (aq)
D. NH4OH (aq)
-
22M.2.sl.TZ1.2e(iii):
Calculate the concentration of hydroxide ions in an ammonia solution with pH = 9.3. Use sections 1 and 2 of the data booklet.
-
22M.2.hl.TZ1.4c(i):
Calculate the concentration of hydroxide ions in an ammonia solution with pH = 9.3. Use sections 1 and 2 of the data booklet.
8.4 Strong and weak acids and bases
- 16N.2.sl.TZ0.2a: Distinguish between a weak acid and a strong acid. Weak acid: Strong acid:
-
17M.1.sl.TZ1.20:
Which 1.0 moldm–3 solution has the highest pH?
A. Ammonium chloride
B. Sulfuric acid
C. Sodium chloride
D. Ammonia
-
17M.2.sl.TZ1.4c:
Hydrazine reacts with water in a similar way to ammonia. Deduce an equation for the reaction of hydrazine with water.
-
17M.2.sl.TZ1.4d:
Outline, using an ionic equation, what is observed when magnesium powder is added to a solution of ammonium chloride.
-
17M.2.hl.TZ2.8b.v:
State a technique other than a pH titration that can be used to detect the equivalence point.
-
17M.3.sl.TZ2.14b.i:
Oceans can act as a carbon sink, removing some CO2(g) from the atmosphere.
CO2(g) CO2(aq)
Aqueous carbon dioxide, CO2(aq), quickly reacts with ocean water in a new equilibrium reaction. Construct the equilibrium equation for this reaction including state symbols.
-
17M.3.sl.TZ2.14b.ii:
Describe how large amounts of CO2 could reduce the pH of the ocean using an equation to support your answer.
-
17M.3.hl.TZ2.4a:
Identify the other product formed.
- 17N.2.hl.TZ0.1e: Suggest why the enthalpy change of neutralization of CH3COOH is less negative than that of HCl.
-
18M.2.hl.TZ1.5a:
Predict, giving a reason, a difference between the reactions of the same concentrations of hydrochloric acid and ethanoic acid with samples of calcium carbonate.
-
18M.2.sl.TZ1.5a:
Predict, giving a reason, a difference between the reactions of the same concentrations of hydrochloric acid and ethanoic acid with samples of calcium carbonate.
- 18M.1.sl.TZ2.20: Which statement is correct? A. A strong acid is a good proton donor and has a strong...
-
18M.2.sl.TZ2.2b.ii:
State and explain the effect on the rate of reaction if ethanoic acid of the same concentration is used in place of hydrochloric acid.
-
18N.1.sl.TZ0.20:
What is the order of increasing pH for the following solutions of the same concentration?
A. HCl (aq) < NH3 (aq) < NaOH (aq) < CH3COOH (aq)
B. CH3COOH (aq) < HCl (aq) < NH3 (aq) < NaOH (aq)
C. HCl (aq) < CH3COOH (aq) < NH3 (aq) < NaOH (aq)
D. NaOH (aq) < NH3 (aq) < CH3COOH (aq) < HCl (aq)
-
18N.2.sl.TZ0.6a:
State the equation for the reaction of each substance with water.
-
18N.2.hl.TZ0.6a.i:
State the equation for the reaction of each substance with water.
-
19M.2.hl.TZ1.5a:
Outline why ethanoic acid is classified as a weak acid.
-
19M.2.hl.TZ2.5a(i):
Distinguish between a weak and strong acid.
Weak acid:
Strong acid:
-
19M.2.sl.TZ1.5a:
Outline why ethanoic acid is classified as a weak acid.
-
19M.2.sl.TZ2.5a(i):
Distinguish between a weak and strong acid.
Weak acid:
Strong acid:
- 19N.2.hl.TZ0.4a(ii): The value of Ka at 298 K for the first dissociation is 5.01 × 10−4. State, giving a reason, the...
-
19N.2.hl.TZ0.4a(iii):
The dissociation of citric acid is an endothermic process. State the effect on the hydrogen ion concentration, [H+], and on Ka, of increasing the temperature.
- 19N.2.hl.TZ0.4b: Outline two laboratory methods of distinguishing between solutions of citric acid and...
-
19N.3.sl.TZ0.16a:
Identify the compound responsible for the acidity of gastric juice, and state whether it is a strong or weak acid.
- 19N.2.sl.TZ0.4a(ii): The value of the equilibrium constant for the first dissociation at 298 K is 5.01 × 10−4. State,...
-
19N.2.sl.TZ0.4a(iii):
The dissociation of citric acid is an endothermic process. State the effect on the hydrogen ion concentration, [H+], and on the equilibrium constant, of increasing the temperature.
- 19N.2.sl.TZ0.4b: Outline one laboratory methods of distinguishing between solutions of citric acid and...
-
20N.1.sl.TZ0.20:
Which of these acids has the weakest conjugate base?
A.
B.
C.
D.
- 20N.2.sl.TZ0.1c(i): Hypochlorous acid is considered a weak acid. Outline what is meant by the term weak acid.
- 20N.2.hl.TZ0.1c(i): Hypochlorous acid is considered a weak acid. Outline what is meant by the term weak acid.
-
21M.2.sl.TZ1.2b(ii):
Saturated aqueous hydrogen sulfide has a concentration of 0.10 mol dm−3 and a pH of 4.0. Demonstrate whether it is a strong or weak acid.
- 21N.1.sl.TZ0.20: Which ions are present in an aqueous solution of Na2CO3? I. HCO3−II. OH−III. CO32− A. I and...
-
22M.1.sl.TZ2.20:
What is the strongest acid in the equation below?
H3AsO4 + H2O H2AsO4− + H3O+ Kc = 4.5 × 10−4
A. H3AsO4
B. H2O
C. H2AsO4−
D. H3O+
-
22M.1.sl.TZ2.30:
20 cm3 of 1 mol dm−3 sulfuric acid was added dropwise to 20 cm3 of 1 mol dm−3 barium hydroxide producing a precipitate of barium sulfate.
H2SO4 (aq) + Ba(OH)2 (aq) → 2H2O (l) + BaSO4 (s)
Which graph represents a plot of conductivity against volume of acid added?
- 22M.2.sl.TZ2.3c(ii): State the meaning of a strong Brønsted–Lowry acid.
- 22M.2.hl.TZ2.6d(ii): State the meaning of a strong Brønsted–Lowry acid.
8.5 Acid deposition
-
16N.2.sl.TZ0.3b:
Suggest why the experiment should be carried out in a fume hood or in a well-ventilated laboratory.
-
17M.2.sl.TZ2.5c:
State the equation for the reaction of NO2 in the atmosphere to produce acid deposition.
-
18M.2.hl.TZ1.5b:
Dissolved carbon dioxide causes unpolluted rain to have a pH of approximately 5, but other dissolved gases can result in a much lower pH. State one environmental effect of acid rain.
-
18M.2.hl.TZ2.2e.i:
Formulate the equation for the reaction of nitrogen dioxide, NO2, with water to form two acids.
-
18M.2.hl.TZ2.2e.ii:
Formulate the equation for the reaction of one of the acids produced in (e)(i) with calcium carbonate.
-
18M.2.sl.TZ1.5b:
Dissolved carbon dioxide causes unpolluted rain to have a pH of approximately 5, but other dissolved gases can result in a much lower pH. State one environmental effect of acid rain.
- 18N.3.sl.TZ0.1a: Outline why the initial reaction should be carried out under a fume hood.
- 18N.3.hl.TZ0.1a: Outline why the initial reaction should be carried out under a fume hood.
-
19M.1.hl.TZ2.25:
What is the major reason why the pH of unpolluted rain is less than 7?
A. methane
B. carbon dioxide
C. nitrogen oxides
D. sulfur dioxide
- 19M.1.sl.TZ1.20: Which is not a source of oxides of sulfur and nitrogen? A. burning coal B. internal combustion...
-
19M.1.sl.TZ2.20:
What is the major reason why the pH of unpolluted rain is less than 7?
A. methane
B. carbon dioxide
C. nitrogen oxides
D. sulfur dioxide
-
20N.1.hl.TZ0.24:
Which of these oxides contribute to acid deposition?
I.
II.
III.A. I and II only
B. I and III only
C. II and III only
D. I, II and III
- 21M.1.sl.TZ2.20: Which causes acid deposition? A. SO2 B. SiO2 C. SrO D. CO2
- 21M.2.sl.TZ1.1d(iii): Suggest why this process might raise environmental concerns.
- 21M.2.hl.TZ1.1e(iii): Suggest why this process might raise environmental concerns.
-
21M.2.sl.TZ2.1f:
Outline how one calcium compound in the lime cycle can reduce a problem caused by acid deposition.
-
21M.2.hl.TZ2.1e:
Outline how one calcium compound in the lime cycle can reduce a problem caused by acid deposition.
- 22M.2.sl.TZ2.3c(i): State the product formed from the reaction of SO3 with water.
-
22M.2.hl.TZ2.6d(i):
State the product formed from the reaction of SO3 with water.
