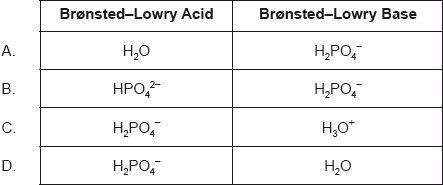
| Date | November 2017 | Marks available | 1 | Reference code | 17N.2.sl.TZ0.5 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Identify | Question number | 5 | Adapted from | N/A |
Question
Many reactions are in a state of equilibrium.
The equations for two acid-base reactions are given below.
HCO3– (aq) + H2O (l) H2CO3 (aq) + OH– (aq)
HCO3– (aq) + H2O (l) CO32– (aq) + H3O+ (aq)
The following reaction was allowed to reach equilibrium at 761 K.
H2 (g) + I2 (g) 2HI (g) ΔHθ < 0
Outline the effect, if any, of each of the following changes on the position of equilibrium, giving a reason in each case.
Identify two different amphiprotic species in the above reactions.
State what is meant by the term conjugate base.
State the conjugate base of the hydroxide ion, OH–.
A student working in the laboratory classified HNO3, H2SO4, H3PO4 and HClO4 as acids based on their pH. He hypothesized that “all acids contain oxygen and hydrogen”.
Evaluate his hypothesis.
Markscheme
Award [1 max] if both effects are correct.
Reason for increasing volume:
Accept “concentration of all reagents reduced by an equal amount so cancels out in Kc expression”.
Accept “affects both forward and backward rates equally”.
HCO3– AND H2O
species that has one less proton/H+ ion «than its conjugate acid»
OR
species that forms its conjugate acid by accepting a proton
OR
species that is formed when an acid donates a proton
Do not accept “differs by one proton/H+ from conjugate acid”.
oxide ion/O2–
insufficient data to make generalization
OR
need to consider a «much» larger number of acids
OR
hypothesis will continue to be tested with new acids to see if it can stand the test of time
«hypothesis is false as» other acids/HCl/HBr/HCN/transition metal ion/BF3 do not contain oxygen
OR
other acids/HCl/HBr/HCN/transition metal ion/BF3 falsify hypothesis
correct inductive reasoning «based on limited sample»
«hypothesis not valid as» it contradicts current/accepted theories/Brønsted-Lowry/Lewis theory
[Max 2 Marks]