| Date | May 2017 | Marks available | 1 | Reference code | 17M.1.hl.TZ1.24 |
| Level | HL | Paper | 1 | Time zone | TZ1 |
| Command term | State | Question number | 24 | Adapted from | N/A |
Question
Which species produced by the successive dissociations of phosphoric acid, H3PO4, are amphiprotic?
A. HPO42− and PO43−
B. H2PO4− and HPO42−
C. H2PO4− and PO43−
D. HPO42− only
Markscheme
B
Examiners report
Syllabus sections
-
18M.1.sl.TZ1.19:
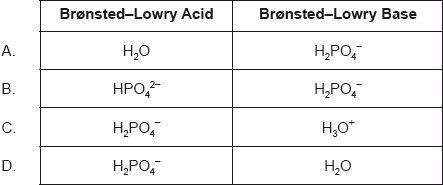
Which classification is correct for the reaction?
H2PO4−(aq) + H2O(l) → HPO42−(aq) + H3O+(aq)
- 22M.2.hl.TZ1.4a: State the relationship between NH4+ and NH3 in terms of the Brønsted–Lowry theory.
-
18N.2.hl.TZ0.6a.i:
State the equation for the reaction of each substance with water.
- 17N.2.sl.TZ0.5b.ii: State what is meant by the term conjugate base.
- 17N.2.sl.TZ0.5b.iii: State the conjugate base of the hydroxide ion, OH–.
- 17N.2.sl.TZ0.5b.i: Identify two different amphiprotic species in the above reactions.
-
17M.2.sl.TZ2.7a.ii:
Identify one conjugate acid-base pair in the reaction.
- 22M.2.sl.TZ2.3c(ii): State the meaning of a strong Brønsted–Lowry acid.
- 17M.1.sl.TZ1.19: Which is an acid-base conjugate pair? A. H3O+ / OH– B. H2SO4 / SO42– C. ...
- 16N.1.sl.TZ0.19: Which species behave as Brønsted–Lowry bases in the following reaction? H2SO4 + HNO3...
-
17M.2.sl.TZ2.7a.i:
Identify the amphiprotic species.
- 19N.2.sl.TZ0.4a(i): Identify a conjugate acid–base pair in the equation.
- 18M.1.hl.TZ1.24: What describes HPO42−? A. Amphiprotic but not amphoteric B. Amphoteric but not...
-
18M.2.hl.TZ1.5c:
Write an equation to show ammonia, NH3, acting as a Brønsted–Lowry base and a different equation to show it acting as a Lewis base.
- 22M.2.hl.TZ2.6d(ii): State the meaning of a strong Brønsted–Lowry acid.
-
18N.2.sl.TZ0.6a:
State the equation for the reaction of each substance with water.
-
21N.2.sl.TZ0.5a:
Formulate an equation for the reaction of one mole of phosphoric acid with one mole of sodium hydroxide.
- 21N.2.sl.TZ0.5d: Outline the reason that sodium hydroxide is considered a Brønsted–Lowry base.
-
17M.1.hl.TZ1.26:
Which species acts as a Lewis and Brønsted–Lowry base?
A. [Al(H2O)6]3+
B. BF3
C. NH4+
D. OH−
-
19M.2.sl.TZ2.5a(ii):
The hydrogencarbonate ion, produced in Equilibrium (2), can also act as an acid.
State the formula of its conjugate base.
- 17M.1.sl.TZ2.20: Which of the following is correct? A. A weak acid is a proton donor and its aqueous...
- 19N.2.hl.TZ0.4a(i): Identify a conjugate acid–base pair in the equation.
- 19N.3.sl.TZ0.1c(i): Justify this hypothesis.
-
17N.2.sl.TZ0.5c:
A student working in the laboratory classified HNO3, H2SO4, H3PO4 and HClO4 as acids based on their pH. He hypothesized that “all acids contain oxygen and hydrogen”.
Evaluate his hypothesis.
-
21N.2.hl.TZ0.5a:
Formulate an equation for the reaction of one mole of phosphoric acid with one mole of sodium hydroxide.
-
17M.2.sl.TZ2.7b.i:
Calculate the amount, in mol, of NaOH(aq) used.
-
19M.2.sl.TZ1.2a:
Draw the structure of the conjugate base of benzoic acid showing all the atoms and all the bonds.
- 19N.1.sl.TZ0.21: Which is an example of an amphiprotic species? A. Al2O3 B. CO32− C. P4O10 D. HPO42−
- 19N.1.sl.TZ0.20: What is the difference between a conjugate Brønsted–Lowry acid–base pair? A. Electron...
-
18N.1.sl.TZ0.19:
Which two species act as Brønsted–Lowry acids in the reaction?
H2PO4− (aq) + OH− (aq) HPO42− (aq) + H2O (l)
A. HPO42− (aq) and OH− (aq)
B. H2PO4− (aq) and HPO42− (aq)
C. HPO42− (aq) and H2O (l)
D. H2PO4− (aq) and H2O (l)
-
20N.2.hl.TZ0.5d:
Calculate the of the conjugate base of ethanoic acid using sections 2 and 21 of the data booklet.
-
19M.2.hl.TZ2.5a(ii):
The hydrogencarbonate ion, produced in Equilibrium (2), can also act as an acid.
State the formula of its conjugate base.
-
18M.2.sl.TZ2.2d:
Outline why H3PO4/HPO42− is not a conjugate acid-base pair.
-
19M.2.hl.TZ2.5e:
The reaction of the hydroxide ion with carbon dioxide and with the hydrogencarbonate ion can be represented by Equations 3 and 4.
Equation (3) OH− (aq) + CO2 (g) → HCO3− (aq)
Equation (4) OH− (aq) + HCO3− (aq) → H2O (l) + CO32− (aq)Discuss how these equations show the difference between a Lewis base and a Brønsted–Lowry base.
Equation (3):
Equation (4):
- 21N.2.hl.TZ0.5d: Outline the reasons that sodium hydroxide is considered a Brønsted–Lowry and Lewis base.
-
21M.1.hl.TZ1.26:
Which is a Lewis acid, but not a Brønsted-Lowry acid?
A.
B.
C.
D.
-
19M.2.hl.TZ1.2d:
Draw the structure of the conjugate base of benzoic acid showing all the atoms and all the bonds.
- 21M.1.sl.TZ2.19: Which cannot act as a Brønsted–Lowry base? A. HPO42− B. H2O C. CH4 D. NH3
- 21M.2.sl.TZ1.2b(i): State the formula of its conjugate base.
-
20N.1.sl.TZ0.20:
Which of these acids has the weakest conjugate base?
A.
B.
C.
D.
-
20N.2.sl.TZ0.1c(ii):
State the formula of the conjugate base of hypochlorous acid.
-
20N.2.hl.TZ0.1c(ii):
State the formula of the conjugate base of hypochlorous acid.
-
20N.1.hl.TZ0.26:
Which species is a Lewis acid but not a Brønsted–Lowry acid?
A.
B.
C.
D.
- 22M.2.sl.TZ1.2e(i): State the relationship between NH4+ and NH3 in terms of the Brønsted–Lowry theory.
-
21N.2.hl.TZ0.5b:
Formulate two equations to show the amphiprotic nature of H2PO4−.
- 21N.1.sl.TZ0.21: What is the conjugate acid of HS−? A. H2S B. S2− C. H2SO3 D. H2SO4
-
21N.2.sl.TZ0.5b:
Formulate two equations to show the amphiprotic nature of H2PO4−.

