| Date | May 2017 | Marks available | 1 | Reference code | 17M.2.sl.TZ2.7 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Identify | Question number | 7 | Adapted from | N/A |
Question
Soluble acids and bases ionize in water.
Sodium hypochlorite ionizes in water.
OCl–(aq) + H2O(l) OH–(aq) + HOCl(aq)
A solution containing 0.510 g of an unknown monoprotic acid, HA, was titrated with 0.100 mol dm–3 NaOH(aq). 25.0 cm3 was required to reach the equivalence point.
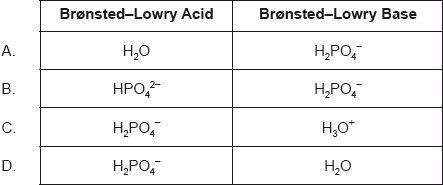
Identify the amphiprotic species.
Identify one conjugate acid-base pair in the reaction.
Calculate the amount, in mol, of NaOH(aq) used.
Calculate the molar mass of the acid.
Calculate [H+] in the NaOH solution.
Markscheme
water/H2O
Accept “hydroxide ion/OH–”.
[1 mark]
[1 mark]
«0.100 moldm–3 x 0.0250 dm3» = 0.00250 «mol»
[1 mark]
«M = =» 204 «gmol–1»
[1 mark]
«1.00 x 10–14 = [H+] x 0.100»
1.00 x 10–13 «moldm–3»
[1 mark]