| Date | May 2018 | Marks available | 1 | Reference code | 18M.2.sl.TZ2.2 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Outline | Question number | 2 | Adapted from | N/A |
Question
Graphing is an important tool in the study of rates of chemical reactions.
Excess hydrochloric acid is added to lumps of calcium carbonate. The graph shows the volume of carbon dioxide gas produced over time.
Sketch a Maxwell–Boltzmann distribution curve for a chemical reaction showing the activation energies with and without a catalyst.
Sketch a curve on the graph to show the volume of gas produced over time if the same mass of crushed calcium carbonate is used instead of lumps. All other conditions remain constant.
State and explain the effect on the rate of reaction if ethanoic acid of the same concentration is used in place of hydrochloric acid.
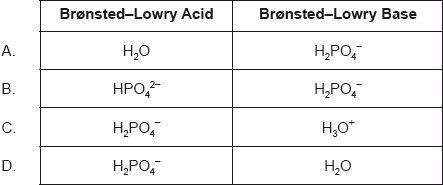
Outline why pH is more widely used than [H+] for measuring relative acidity.
Outline why H3PO4/HPO42− is not a conjugate acid-base pair.
Markscheme
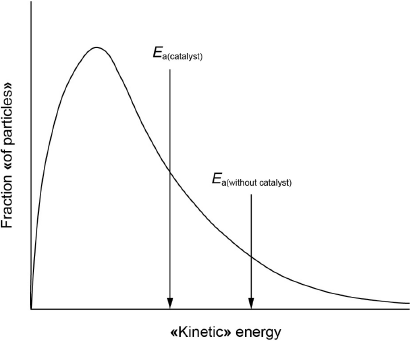
both axes correctly labelled
correct shape of curve starting at origin
Ea(catalyst) < Ea(without catalyst) on x-axis
M1:
Accept “speed” for x-axis label.
Accept “number of particles”, “N”, “frequency” or “probability «density»” for y-axis label.
Do not accept “potential energy” for x-axis label.
M2:
Do not accept a curve that touches the x-axis at high energy.
Do not award M2 if two curves are drawn.
M3:
Ignore any shading under the curve.
[3 marks]
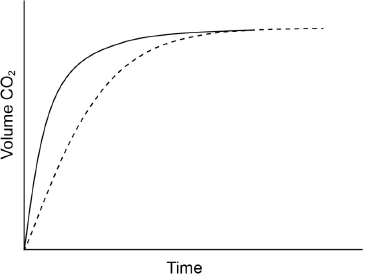
curve starting from origin with steeper gradient AND reaching same maximum volume
[1 mark]
rate decreases
OR
slower reaction
«ethanoic acid» partially dissociated/ionized «in solution/water»
OR
lower [H+]
Accept “weak acid” or “higher pH”.
[2 marks]
«pH» converts «wide range of [H+]» into simple «log» scale/numbers
OR
«pH» avoids need for exponential/scientific notation
OR
«pH» converts small numbers into values «typically» between 0/1 and 14
OR
«pH» allows easy comparison of values of [H+]
Accept “uses values between 0/1 and 14”.
Do not accept “easier to use”.
Do not accept “easier for calculations”.
[1 mark]
«species» do not differ by a «single» proton/H+
OR
conjugate base of H3PO4 is H2PO4– «not HPO42–»
OR
conjugate acid of HPO42– is H2PO4– «not H3PO4»
Do not accept “hydrogen/H” for “H+/proton”.
[1 mark]