| Date | May 2014 | Marks available | 1 | Reference code | 14M.2.hl.TZ2.1 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Suggest | Question number | 1 | Adapted from | N/A |
Question
A class studied the equilibrium established when ethanoic acid and ethanol react together in the presence of a strong acid, using propanone as an inert solvent. The equation is given below.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}} + {{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{OH}} \rightleftharpoons {\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}} + {{\text{H}}_{\text{2}}}{\text{O}}\]
One group made the following initial mixture:
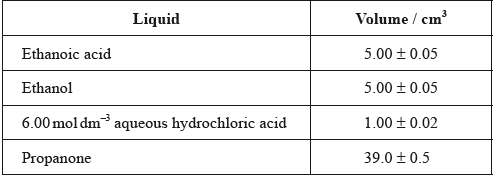
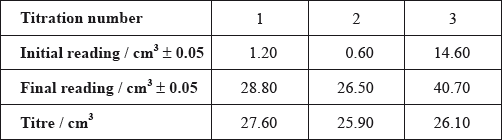
After one week, a \(5.00 \pm 0.05{\text{ c}}{{\text{m}}^{\text{3}}}\) sample of the final equilibrium mixture was pipetted out and titrated with \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide to determine the amount of ethanoic acid remaining. The following titration results were obtained:
The density of ethanoic acid is \({\text{1.05 g}}\,{\text{c}}{{\text{m}}^{ - 3}}\). Determine the amount, in mol, of ethanoic acid present in the initial mixture.
The concentration of ethanoic acid can be calculated as \({\text{1.748 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\). Determine the percentage uncertainty of this value. (Neglect any uncertainty in the density and the molar mass.)
Calculate the absolute uncertainty of the titre for Titration 1 (\({\text{27.60 c}}{{\text{m}}^3}\)).
Suggest the average volume of alkali, required to neutralize the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample, that the student should use.
\({\text{3.00 c}}{{\text{m}}^{\text{3}}}\) of the \({\text{0.200 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) aqueous sodium hydroxide reacted with the hydrochloric acid present in the \({\text{5.00 c}}{{\text{m}}^{\text{3}}}\) sample. Determine the concentration of ethanoic acid in the final equilibrium mixture.
Deduce the equilibrium constant expression for the reaction.
The other concentrations in the equilibrium mixture were calculated as follows:
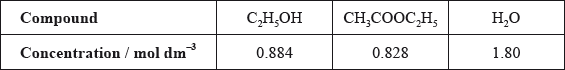
Use these data, along with your answer to part (iii), to determine the value of the equilibrium constant. (If you did not obtain an answer to part (iii), assume the concentrations of ethanol and ethanoic acid are equal, although this is not the case.)
Outline how you could establish that the system had reached equilibrium at the end of one week.
Outline why changing the temperature has only a very small effect on the value of the equilibrium constant for this equilibrium.
Outline how adding some ethyl ethanoate to the initial mixture would affect the amount of ethanoic acid converted to product.
Propanone is used as the solvent because one compound involved in the equilibrium is insoluble in water. Identify this compound and explain why it is insoluble in water.
Suggest one other reason why using water as a solvent would make the experiment less successful.
Markscheme
\({\text{M(C}}{{\text{H}}_{\text{3}}}{\text{COOH)}}\left( { = (4 \times 1.01) + (2 \times 12.01) + (2 \times 16.00)} \right) = 60.06{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ - 1}})\);
Accept 60 (g mol–1).
\({\text{mass (C}}{{\text{H}}_3}{\text{COOH) }}( = 5.00 \times 1.05) = 5.25{\text{ (g)}}\);
\(\frac{{5.25}}{{60.06}} = 0.0874{\text{ (mol)}}\);
Award [3] for correct final answer.
Accept 0.0875 (comes from using Mr = 60 g mol–1).
percentage uncertainty in volume of ethanoic acid \( = 100 \times \frac{{0.05}}{{5.00}}{\text{ }} = 1\% \);
percentage uncertainty in total volume \( = 100 \times \frac{{0.62}}{{50}} = 1.24\% \);
total percentage uncertainty \( = 1 + 1.24 = 2.24\% \);
Accept rounding down to 2.2/2%.
\( \pm 0.1/0.10{\text{ }}({\text{c}}{{\text{m}}^3})\);
Do not accept without ±.
\({\text{26.00 (c}}{{\text{m}}^{\text{3}}}{\text{)}}\);
\(26.00 - 3.00 = 23.00{\text{ }}({\text{c}}{{\text{m}}^3})\);
If other methods used, award M1 for calculating amount of NaOH reacting with CH3COOH.
\(0.200 \times \frac{{23.00}}{{5.00}} = 0.920{\text{ }}({\text{mol}}\,{\text{d}}{{\text{m}}^{ - 3}})\);
Award [2] for correct final answer.
If (ii) given as mean titre (26.5 cm3) then ECF answer comes to 0.94 (mol dm–3).
\(({K_{\text{c}}} = )\frac{{{\text{[C}}{{\text{H}}_3}{\text{COO}}{{\text{C}}_2}{{\text{H}}_5}{\text{][}}{{\text{H}}_2}{\text{O]}}}}{{{\text{[}}{{\text{C}}_2}{{\text{H}}_5}{\text{OH][C}}{{\text{H}}_3}{\text{COOH]}}}}\);
Do not penalize minor errors in formulas.
Accept \(({K_{\text{c}}} = )\frac{{{\text{[}}esther{\text{][}}water{\text{]}}}}{{[ethanol/alcohol{\text{][(}}ethanoic{\text{) }}acid{\text{]}}}}\).
\(({K_c} = )\frac{{0.828 \times 1.80}}{{0.884 \times 0.920}} = 1.83\);
If assumed [CH3COOH] = 0.884 mol dm-3, answer is 1.91 – allow this even if an answer was obtained for (iii).
If (ii) given as mean titre (26.5 cm3) then ECF answer comes to 1.79.
repeat the titration a day/week later (and result should be the same) / OWTTE;
Accept “concentrations/physical properties/macroscopic properties of the system do not change”.
enthalpy change/\(\Delta H\) for the reaction is (very) small / OWTTE;
decreases (the amount of ethanoic acid converted);
Accept “increases amount of ethanoic acid present at equilibrium” / OWTTE.
(adding product) shifts position of equilibrium towards reactants/LHS / increases
the rate of the reverse reaction / OWTTE;
ethyl ethanoate/\({\text{C}}{{\text{H}}_{\text{3}}}{\text{COO}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\)/ester;
forms only weak hydrogen bonds (to water);
Allow “does not hydrogen bond to water” / “hydrocarbon sections too long” / OWTTE.
M2 can only be given only if M1 correct.
(large excess of) water will shift the position of equilibrium (far to the left) / OWTTE;
Accept any other chemically sound response, such as “dissociation of ethanoic acid would affect equilibrium”.
Examiners report
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.
Generally candidates found this question quite challenging and some left quite a number of parts unanswered. The tradition is that the first question on the paper is a data response question, which often addresses many aspects of the syllabus, and unfortunately candidates, especially those of average or below average ability, seem to have difficulty in tackling questions of this nature. One other issue with data response questions is that, of necessity, the data appears at the beginning of the question whilst, mainly because of the space left for candidates to answer, the later parts of the question referring to these data may not appear until a number of pages into the paper.
Part (a) concerning density, volume and amount of substance was generally reasonably well answered, but the following parts, concerning uncertainties, were rarely answered correctly and a number confused precision (uncertainty, either absolute or as a percentage) and accuracy (percentage error in the value obtained). Many candidates also seemed to lack experimental common sense, simply taking an average that included an initial titre that was much larger than the concordant second and third titres, rather than excluding it. This lack of experimental “know how” was also evident in responses to (c) (iii) where it was unusual for the approach to the question to indicate the candidate had realised that the alkali was neutralising two different acids (HCl and \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COOH}}\)) and again in part (d) where it was rare for the response to outline a practical solution to the problem, though quite a number of candidates suggested that the pH would become constant, presumably not realising that the pH would be dominated by the HCl catalyst. Most students could however carry out the more routine tasks of writing an equilibrium constant expression and determining its value from the data given. Many candidates were aware of Le Chatelier effects on the position of equilibrium, but a significant number failed to use this information to answer the question actually asked and the unusual approach to the effect of temperature disconcerted many. Whilst most students managed to identify the ester as the component of the mixture that was insoluble in water, the reasons given were usually couched in terms of the polarity of the molecule (many quite polar molecules, halogenoalkanes for example, are insoluble in water) rather than its inability to form strong hydrogen bonds to water, which is the critical factor. Quite a number of students came up with a valid reason why water would not be a suitable solvent, though some students appeared to have overlooked the fact the question stated “other reason”.

