| Date | May 2013 | Marks available | 2 | Reference code | 13M.2.sl.TZ1.1 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Calculate | Question number | 1 | Adapted from | N/A |
Question
A student decided to determine the molecular mass of a solid monoprotic acid, HA, by titrating a solution of a known mass of the acid.
The following recordings were made.
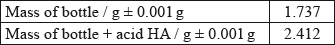
Calculate the mass of the acid and determine its absolute and percentage uncertainty.
This known mass of acid, HA, was then dissolved in distilled water to form a \({\text{100.0 c}}{{\text{m}}^{\text{3}}}\) solution in a volumetric flask. A \({\text{25.0 c}}{{\text{m}}^{\text{3}}}\) sample of this solution reacted with \({\text{12.1 c}}{{\text{m}}^{\text{3}}}\) of a \({\text{0.100 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) NaOH solution. Calculate the molar mass of the acid.
The percentage composition of HA is 70.56% carbon, 23.50% oxygen and 5.94% hydrogen. Determine its empirical formula.
A solution of HA is a weak acid. Distinguish between a weak acid and a strong acid.
Describe an experiment, other than measuring the pH, to distinguish HA from a strong acid of the same concentration and describe what would be observed.
Markscheme
0.675 (g) ± 0.002 (g);
Percentage uncertainty: 0.3%;
Accept answers correct to one, two or three significant figures for percentage uncertainty.
In 25.0 cm3: \({n_{{\text{HA}}}} = 1.21 \times {10^{ - 3}}{\text{ (mol)}}\);
In 100 cm3: \({n_{{\text{HA}}}} = 4.84 \times {10^{ - 3}}{\text{ (mol)}}\);
\({\text{M }}\left( { = \frac{{0.675}}{{4.84 \times {{10}^{ - 3}}}}} \right) = 139{\text{ (g}}\,{\text{mo}}{{\text{l}}^{ - 1}}{\text{)}}\);
Award [3] for correct final answer.
Accept suitable alternative methods.
\({n_{\text{C}}}:{\text{ }}\left( {\frac{{70.56}}{{12.01}} = } \right){\text{ }}5.88\) and \({n_{\text{O}}}:{\text{ }}\left( {\frac{{23.50}}{{16}} = } \right){\text{ }}1.47\) and \({n_{\text{H}}}:{\text{ }}\left( {\frac{{5.94}}{{1.01}} = } \right){\text{ }}5.88\)
\({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{4}}}{\text{O}}\);
Award [2] for correct final answer.
Accept answers using integer values of molar mass.
weak acids partially dissociated/ionized and strong acids completely dissociated/ionized (in solution/water) / OWTTE;
strong acids have greater electrical conductivity / weak acids have lower electrical conductivity;
OR
adding a reactive metal / carbonate / hydrogen carbonate;
Accept correct example.
stronger effervescence with strong acids / weaker with weak acids / OWTTE;
OR
adding a strong base;
Accept correct example.
strong acid would increase more in temperature / weak acids increase less in temperature;
Examiners report
Many students lost easy marks as they forgot to propagate uncertainties.
Many candidates struggled with the concept of mole and the dilution factor added to the difficulty.
Most students determined the empirical formula correctly.
Weak and strong acids were generally correctly defined, though sometimes they were defined in terms of pH.
The conductivity test appeared frequently and was well described. Many candidates used a strong based, but then went on to describe a titration method.

