| Date | November 2011 | Marks available | 1 | Reference code | 11N.2.sl.TZ0.1 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Calculate | Question number | 1 | Adapted from | N/A |
Question
Airbags are an important safety feature in vehicles. Sodium azide, potassium nitrate and silicon dioxide have been used in one design of airbag.

Sodium azide, a toxic compound, undergoes the following decomposition reaction under certain conditions.
\[{\text{2Na}}{{\text{N}}_{\text{3}}}{\text{(s)}} \to {\text{2Na(s)}} + {\text{3}}{{\text{N}}_{\text{2}}}{\text{(g)}}\]
Two students looked at data in a simulated computer-based experiment to determine the volume of nitrogen generated in an airbag.
Using the simulation programme, the students entered the following data into the computer.
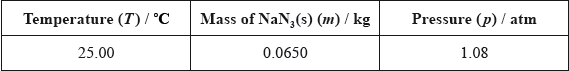
The chemistry of the airbag was found to involve three reactions. The first reaction involves the decomposition of sodium azide to form sodium and nitrogen. In the second reaction, potassium nitrate reacts with sodium.
\[{\text{2KN}}{{\text{O}}_3}{\text{(s)}} + {\text{10Na(s)}} \to {{\text{K}}_2}{\text{O(s)}} + {\text{5N}}{{\text{a}}_2}{\text{O(s)}} + {{\text{N}}_2}{\text{(g)}}\]
An airbag inflates very quickly.
Sodium azide involves ionic bonding, and metallic bonding is present in sodium. Describe ionic and metallic bonding.
State the number of significant figures for the temperature, mass and pressure data.
T:
m:
p:
Calculate the amount, in mol, of sodium azide present.
Determine the volume of nitrogen gas, in \({\text{d}}{{\text{m}}^{\text{3}}}\), produced under these conditions based on this reaction.
Suggest why it is necessary for sodium to be removed by this reaction.
The metal oxides from the second reaction then react with silicon dioxide to form a silicate in the third reaction.
\[{{\text{K}}_2}{\text{O(s)}} + {\text{N}}{{\text{a}}_2}{\text{O(s)}} + {\text{Si}}{{\text{O}}_2}{\text{(s)}} \to {\text{N}}{{\text{a}}_2}{{\text{K}}_2}{\text{Si}}{{\text{O}}_4}{\text{(s)}}\]
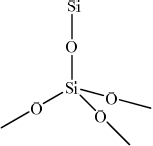
Draw the structure of silicon dioxide and state the type of bonding present.
Structure:
Bonding:
It takes just 0.0400 seconds to produce nitrogen gas in the simulation. Calculate the average rate of formation of nitrogen in (b) (iii) and state its units.
The students also discovered that a small increase in temperature (e.g. 10 °C) causes a large increase (e.g. doubling) in the rate of this reaction. State one reason for this.
Markscheme
Ionic:
(electrostatic) attraction between oppositely charged ions/cations and anions/positive and negative ions;
Do not accept answers such as compounds containing metal and non-metal are ionic.
Metallic:
(electrostatic attraction between lattice of) positive ions/cations/nuclei and delocalized electrons / (bed of) positive ions/cations/nuclei in sea of electrons / OWTTE;
T: 4 and m: 3 and p: 3;
\(n = (65.0/65.02) = 1.00{\text{ (mol)}}\);
No penalty for using whole number atomic masses.
\(n{\text{(}}{{\text{N}}_{\text{2}}}{\text{)}} = \left( {\frac{3}{2} \times 1.00 = } \right){\text{ }}1.50{\text{ (mol)}}\);
\(T = \left( {(25.00 + 273.15) = } \right){\text{ }}298.15{\text{ K}}/(25.00 + 273) = 298{\text{ K}}\);
\(p = 1.08 \times 1.01 \times {10^5}{\text{ Pa}}/1.08 \times 1.01 \times {10^2}{\text{ kPa}}/1.09 \times {10^5}{\text{ Pa}}/1.09 \times {10^2}{\text{ kPa}}\);
\(V = \frac{{nRT}}{p} = \frac{{({{10}^3})(1.50)(8.31)(298.15/298)}}{{(1.08 \times 1.01 \times {{10}^5})}} = 34.1{\text{ (d}}{{\text{m}}^{\text{3}}}{\text{)}}\);
Award [4] for correct final answer.
Award [3 max] for 0.0341 (dm3) or 22.7 (dm3).
Award [3 max] for 34.4 (dm3).
Award [2 max] for 22.9 (dm3).
Award [2 max] for 0.0227 (dm3).
Award [2 max] for 0.034 (dm3).
sodium could react violently with any moisture present / sodium is (potentially) explosive / sodium (is dangerous since it is flammable when it) forms hydrogen on contact with water / OWTTE;
Do not accept answers such as sodium is dangerous or sodium is too reactive.
Structure:
drawing of giant structure showing tetrahedrally arranged silicon;
Minimum information required for mark is Si and 4 O atoms, in a tetrahedral arrangement (not 90° bond angles) but with each of the 4 O atoms showing an extension bond.
Bonding:
(giant/network/3D) covalent;
\(\left( {\frac{{34.1}}{{0.0400}}} \right) = 853{\text{ d}}{{\text{m}}^{\text{3}}}{{\text{s}}^{ - 1}}/\left( {\frac{{1.50}}{{0.0400}}} \right) = 37.5{\text{ mol}}\,{{\text{s}}^{ - 1}}\);
Accept 851 dm3s–1.
Units required for mark.
more energetic collisions / more species have energy \( \geqslant {E_{\text{a}}}\);
Allow more frequent collisions / species collide more often.
Examiners report
Question 1 tested a number of concepts and very few students were able to gain all the marks available. Part (a) was fairly well done and students could explain ionic and metallic bonding although weak students did not explain the bonding but simply stated that ionic was between metal and non metal etc.
Surprisingly in part (b) (i) a number of students could not state the number of significant figures and many stated that 25.00 was 2 SF instead of 4.
Part (b) (ii) required the calculation of the amount of substance in moles, and was generally well done although some did not realise the value was in kg and so had a value 1000 times too small.
In part (b) (iii) a number of students lost marks for forgetting to convert temperature or pressure and also to multiply the amount by 1.5. Also many forgot to convert the pressure into kPa if they wanted their answer in \({\text{d}}{{\text{m}}^{\text{3}}}\). However, most students could obtain at least one of the marks available.
In part (c) (i) many did not relate the removal of sodium to the potential for it to react with water and instead gave a far too vague of answer that it was reactive. However, the very best students were able to answer this hypothesis type question and stated that sodium reacts with water. This proved a good discriminator at the top end of the candidature.
Part (c)(ii) was very poorly answered and the majority of students believed that \({\text{Si}}{{\text{O}}_{\text{2}}}\) had a similar structure to \({\text{C}}{{\text{O}}_{\text{2}}}\). The very few students that drew a giant structure often did not then show a tetrahedral arrangement of the atoms, however most did realise that the bonding was covalent.
Part (d) was generally well answered and most students calculated a rate from their results although some lost the mark for incorrect or absent units.
Most students could then successfully explain why the rate increased with temperature. However a minority forgot to refer to time (i.e. more frequent) in relation to collisions.

