| Date | May 2010 | Marks available | 10 | Reference code | 10M.2.sl.TZ1.1 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Calculate, Deduce, Describe, Determine, and Identify | Question number | 1 | Adapted from | N/A |
Question
Brass is a copper containing alloy with many uses. An analysis is carried out to determine the percentage of copper present in three identical samples of brass. The reactions involved in this analysis are shown below.
\[\begin{array}{*{20}{l}} {{\text{Step 1: Cu(s)}} + {\text{2HN}}{{\text{O}}_3}{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2N}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}}} \\ {{\text{Step 2: 4}}{{\text{I}}^ - }{\text{(aq)}} + {\text{2C}}{{\text{u}}^{2 + }}{\text{(aq)}} \to {\text{2CuI(s)}} + {{\text{I}}_2}{\text{(aq)}}} \\ {{\text{Step 3: }}{{\text{I}}_2}{\text{(aq)}} + {\text{2}}{{\text{S}}_2}{\text{O}}_3^{2 - }{\text{(aq)}} \to {\text{2}}{{\text{I}}^ - }{\text{(aq)}} + {{\text{S}}_4}{\text{O}}_6^{2 - }{\text{(aq)}}} \end{array}\]
(a) (i) Deduce the change in the oxidation numbers of copper and nitrogen in step 1.
Copper:
Nitrogen:
(ii) Identify the oxidizing agent in step 1.
(b) A student carried out this experiment three times, with three identical small brass nails, and obtained the following results.
\[{\text{Mass of brass}} = 0.456{\text{ g}} \pm 0.001{\text{ g}}\]
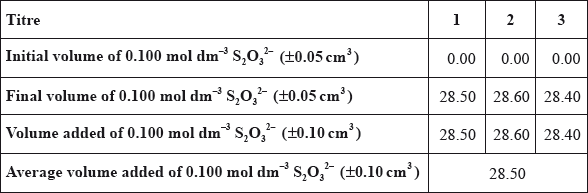
(i) Calculate the average amount, in mol, of \({{\text{S}}_{\text{2}}}{\text{O}}_3^{2 - }\) added in step 3.
(ii) Calculate the amount, in mol, of copper present in the brass.
(iii) Calculate the mass of copper in the brass.
(iv) Calculate the percentage by mass of copper in the brass.
(v) The manufacturers claim that the sample of brass contains 44.2% copper by mass. Determine the percentage error in the result.
(c) With reference to its metallic structure, describe how brass conducts electricity.
Markscheme
(a) (i) Copper:
0 to +2 / increases by 2 / +2 / 2+;
Allow zero/nought for 0.
Nitrogen:
+5 to +4 / decreases by 1 / –1 / 1–;
Penalize missing + sign or incorrect notation such as 2+, 2+ or II, once only.
(ii) nitric acid/\({\text{HN}}{{\text{O}}_{\text{3}}}\) / \({\text{NO}}_3^ - \)/nitrate;
Allow nitrogen from nitric acid/nitrate but not just nitrogen.
(b) (i) \(0.100 \times 0.0285\);
\(2.85 \times {10^{ - 3}}{\text{ (mol)}}\);
Award [2] for correct final answer.
(ii) \(2.85 \times {10^{ - 3}}{\text{ (mol)}}\);
(iii) \(63.55 \times 2.85 \times {10^{ - 3}} = 0.181{\text{ g}}\);
Allow 63.5.
(iv) \(\left( {\frac{{0.181}}{{0.456}} \times 100 = } \right){\text{ }}39.7\% \);
(v) \(\left( {\frac{{44.2 - 39.7}}{{44.2}} \times 100 = } \right){\text{ }}10.2\% \);
Allow 11.3% i.e. percentage obtained in (iv) is used to divide instead of 44.2%.
(c) Brass has:
delocalized electrons / sea of mobile electrons / sea of electrons free to move;
No mark for just “mobile electrons”.
Examiners report
There were several G2 comments on this question, all of which claimed that the question was difficult for SL candidates especially as a three-step reaction process was involved. Certainly some of the weaker candidates struggled with this question, but with the application of ECF marks, most candidates should have been able to score the majority of marks in the question. What was more worrying was the large number of candidates who scored zero or close to zero marks on Q.1, which meant they had little idea of a titration from their exposure to laboratory work in the programme as a whole.
In (a) (i), most candidates showed a reasonable understanding of oxidation numbers, but relatively few scored full marks as they did not read the question which asked explicitly for the change in oxidation numbers. A number also incorrectly wrote 5+ going to 4+ instead of +5 going to +4 i.e. they mixed up charges with oxidation numbers. In the oxidizing agent question in part (ii), the most common mistake was candidates writing nitrogen, instead of the nitric acid, which is the agent involved. In (b), candidates typically either did very well or scored almost no marks at all. In (i), a number of candidates did not convert to dm3 and some did not use the average volume in their calculations, again failing to read the question carefully. (c) however was well answered, though some candidates made reference to the ions as charge carriers rather than giving a description of delocalized electrons. Other candidates stated just mobile electrons instead of stating sea of mobile electrons which was required for the mark.

