| Date | May 2018 | Marks available | 1 | Reference code | 18M.1.hl.TZ2.20 |
| Level | HL | Paper | 1 | Time zone | TZ2 |
| Command term | Apply | Question number | 20 | Adapted from | N/A |
Question
When X reacts with Y to give Z, the following graph is plotted. What can be deduced from the graph?
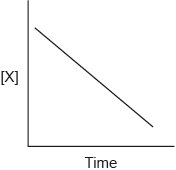
A. The concentration of X is directly proportional to time.
B. The reaction is first order overall.
C. The reaction is zero order with respect to X.
D. The reaction is first order with respect to X.
Markscheme
C
Examiners report
[N/A]
Syllabus sections
Additional higher level (AHL) » Topic 16: Chemical kinetics » 16.1 Rate expression and reaction mechanism
Show 88 related questions
- 17N.2.hl.TZ0.2e.iii: Calculate the value of the rate constant at 263 K.
- 17N.2.hl.TZ0.2e.ii: State the rate expression for the reaction.
- 17N.2.hl.TZ0.2e.i: Deduce the order of reaction with respect to Cl2 and NO.
- 17N.1.hl.TZ0.22: Which pair of statements explains the increase in rate of reaction when the temperature...
- 17N.1.hl.TZ0.21: The rate expression for the reaction X (g) + 2Y (g) → 3Z (g) is rate = k[X]0 [Y]2 By which...
- 17M.2.hl.TZ2.5b.v: Sketch the relationship between the rate of reaction and the concentration of NO2.
- 17M.2.hl.TZ2.5b.iii: State one method that can be used to measure the rate for this reaction.
- 17M.2.hl.TZ2.5b.ii: The following mechanism is proposed for the...
- 17M.2.hl.TZ2.5b.i: State the rate expression for the reaction.
- 17M.1.hl.TZ2.21: Which is correct about reaction mechanisms? A. A species that is zero order does not...
- 17M.1.hl.TZ2.12: Which is the first step in the CFC-catalysed destruction of ozone in UV light? A. CCl2F2...
- 17M.2.hl.TZ1.1d: When the reaction is carried out in the absence of acid the following graph is...
- 17M.2.hl.TZ1.1c: When the concentration of iodine is varied, while keeping the concentrations of acid and...
- 17M.2.hl.TZ1.1b: The student then carried out the experiment at other acid concentrations with all...
- 17M.1.hl.TZ1.21: What are the units for the rate constant, k, in the expression? Rate = k [X]2[Y] A. ...
- 17M.1.hl.TZ1.20: The table gives rate data for the reaction in a suitable solvent. C4H9Br + OH− → C4H9OH +...
- 16N.2.hl.TZ0.3e: (i) Using the graph, explain the order of reaction with respect to sodium thiosulfate. (ii)...
- 16N.1.hl.TZ0.22: Decomposition of hydrogen peroxide in an aqueous solution proceeds as follows. 2H2O2(aq) →...
- 16N.1.hl.TZ0.21: Which statement describes the characteristics of a transition state relative to the potential...
- 16M.2.hl.TZ0.3a: (i) State the equation for the overall reaction. (ii) Deduce the rate expression consistent...
- 16M.1.hl.TZ0.22: Which pair of graphs...
- 16M.1.hl.TZ0.21: The data shows the effect of...
- 15M.1.hl.TZ1.20: Which statement about a first-order reaction is correct? A. The reactant concentration...
- 15M.1.hl.TZ1.21: Consider the rate expression: \[{\text{Rate}} = k{\text{[X][Y]}}\] Which change decreases...
- 15M.1.hl.TZ2.21: The hydrolysis of tertiary bromoalkanes with a warm dilute aqueous sodium hydroxide solution...
- 15M.1.hl.TZ2.20: What are the units of the rate constant for a zero-order reaction? A. s B. ...
- 15M.2.hl.TZ1.7c.i: Determine the orders of reaction of the reactants and the overall rate expression for the...
- 15M.2.hl.TZ1.7c.ii: Determine the rate constant, \(k\), with its units, using the data from experiment 3.
- 15M.2.hl.TZ1.7c.iii: Identify the molecularity of the rate-determining step in this reaction.
- 15M.2.hl.TZ2.2c.i: Identify the rate-determining step.
- 15M.2.hl.TZ2.2c.ii: A student hypothesized that the order of reaction with respect to \({{\text{H}}_{\text{2}}}\)...
- 14M.1.hl.TZ1.20: X and Y react according to the equation \({\text{2X}} + {\text{Y}} \to {\text{Z}}\). The...
- 14M.1.hl.TZ2.21: Which combination shows a second-order rate expression with the correct rate constant units?
- 14M.2.hl.TZ1.2b: State the rate expression for this reaction.
- 14M.2.hl.TZ1.2c: Determine the value of the rate constant, \(k\), and state its units.
- 14M.2.hl.TZ1.2d: State an equation for a possible rate-determining step for the reaction.
- 14M.2.hl.TZ2.6a: (i) State the volumes of the liquids that should be mixed. (ii) State why it is...
- 14M.2.hl.TZ2.6b: (i) Deduce the rate expression of this mechanism. (ii) The results of an...
- 14N.1.hl.TZ0.20: Consider the following reaction between nitrogen monoxide and...
- 14N.2.hl.TZ0.11c: (i) In some reactions, increasing the concentration of a reactant does not increase the...
- 14N.2.hl.TZ0.11d: Sketch a graph of rate constant \((k)\) versus temperature.
- 13N.1.hl.TZ0.22: Consider the following proposed two-step reaction mechanism at temperature T. Step 1: ...
- 13N.1.hl.TZ0.21: The following experimental rate data were obtained for a reaction carried out at temperature...
- 13N.2.hl.TZ0.8e.ii: State the rate expression for this reaction and the units of the rate constant.
- 13M.1.hl.TZ1.21: Which graph best represents a second-order reaction?
- 13M.1.hl.TZ1.20: For the gas phase reaction: \[{\text{A(g)}} + {\text{B(g)}} \to {\text{C(g)}}\] the...
- 13M.2.hl.TZ1.9c.i: Deduce the order of reaction with respect to...
- 13M.2.hl.TZ1.9c.ii: Deduce the rate expression.
- 13M.2.hl.TZ1.9c.iii: Based on the rate expression obtained in (c) (ii) state the units of the rate constant, \(k\).
- 13M.1.hl.TZ2.20: Experimental data shows that a reaction in which Y is a reactant is first order with respect...
- 13M.1.hl.TZ2.21: Which statement about a reaction best describes the relationship between the temperature,...
- 13M.1.hl.TZ2.22: Carbon monoxide and nitrogen dioxide react to form carbon dioxide and nitrogen monoxide...
- 13M.2.hl.TZ2.2b: State how the rate constant, k , varies with temperature, T.
- 13M.2.hl.TZ2.2c: Determine the activation energy, \({E_{\text{a}}}\), correct to three significant figures and...
- 12N.2.hl.TZ0.6d: (i) Deduce the rate expression. (ii) Determine the rate constant, \(k\), and state...
- 12N.2.hl.TZ0.6c: (i) Concentration of reactant X against time for a zero-order reaction. (ii) Rate...
- 10N.1.hl.TZ0.20: Consider the following reaction. \[{\text{2P}} + {\text{Q}} \to {\text{R}} +...
- 10N.2.hl.TZ0.5g: (i) Deduce the rate expression for the reaction. (ii) Determine the value of the...
- 09N.2.hl.TZ0.6a.i: State the rate expression for the forward reaction.
- 09N.2.hl.TZ0.6b: Consider the following...
- 10M.2.hl.TZ1.2b: (i) Deduce the order of reaction for each substance and the rate expression from the...
- 10M.2.hl.TZ1.2c: Using the data from Experiment 1, determine the concentration of the substances used and the...
- 10M.1.hl.TZ2.21: The following data were obtained for the reaction between gases A and B. Which...
- 09M.1.hl.TZ2.21: Consider the following...
- 09M.1.hl.TZ2.22: The rate expression for a reaction is: \[{\text{rate}} = k{\text{[X][Y]}}\] Which statement...
- 09M.1.hl.TZ2.23: Consider the following reaction...
- 09M.2.hl.TZ2.7c.i: Identify the rate-determining step.
- 09M.2.hl.TZ2.7c.ii: Identify the intermediate involved in the reaction.
- 09M.2.hl.TZ2.7b.i: The kinetics of the reaction were studied at this temperature. The table shows the initial...
- 09M.2.hl.TZ2.7b.iii: Determine the value of the rate constant for the reaction from Experiment 3 and state its units.
- 09M.2.hl.TZ2.7b.ii: Deduce the rate expression for the reaction.
- 11M.1.hl.TZ1.23: Which step is the rate-determining step of a reaction? A. The step with the lowest...
- 11M.1.hl.TZ1.21: Bromine and nitrogen(II) oxide react according to the following...
- 11M.2.hl.TZ1.2c: The rate expression for this reaction is rate...
- 11M.1.hl.TZ2.22: Consider the following...
- 11M.1.hl.TZ2.21: The rate information below was obtained for the following reaction at a constant...
- 11M.2.hl.TZ2.3b.ii: Deduce the units for the rate constant \(k\).
- 12M.1.hl.TZ2.19: Which graph represents a reaction that is second order with respect to X for the reaction X...
- 11N.1.hl.TZ0.22: Which graph represents a reaction that is first order with respect to reactant A.
- 11N.2.hl.TZ0.8f.i: Identify the intermediate in the reaction.
- 11N.2.hl.TZ0.8e.ii: The table below shows initial rates of reaction for different concentrations of each reactant...
- 11N.2.hl.TZ0.8f.ii: The observed rate expression is...
- 11N.2.hl.TZ0.8g: The following two-step mechanism has been suggested for the reaction of...
- 18M.2.hl.TZ1.4b.v: Calculate the rate constant of the reaction, stating its units.
- 18M.2.hl.TZ1.4b.iv: Deduce the rate expression for the reaction.
- 18M.1.hl.TZ2.21: Which statement is correct? A. The value of the rate constant, k, is independent of...
- 18M.1.hl.TZ1.20: The reaction between NO2 and F2 gives the following rate data at a certain...
- 18M.1.hl.TZ1.19: What are correct labels for the Maxwell−Boltzmann energy distribution curves?

