| Date | November 2013 | Marks available | 2 | Reference code | 13N.2.hl.TZ0.8 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | State | Question number | 8 | Adapted from | N/A |
Question
2-methylbutan-2-ol, \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{C(OH)C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{3}}}\), is a liquid with a smell of camphor that was formerly used as a sedative. One way of producing it starts with 2-methylbut-2-ene.
As well as 2-methylbutan-2-ol, the reaction also produces a small quantity of an optically active isomer, X.
2-methylbutan-2-ol can also be produced by the hydrolysis of 2-chloro-2-methylbutane, \({{\text{(C}}{{\text{H}}_{\text{3}}}{\text{)}}_{\text{2}}}{\text{CCl}}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\), with aqueous sodium hydroxide.
2-chloro-2-methylbutane contains some molecules with a molar mass of approximately \({\text{106 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\) and some with a molar mass of approximately \({\text{108 g}}\,{\text{mo}}{{\text{l}}^{ - 1}}\).
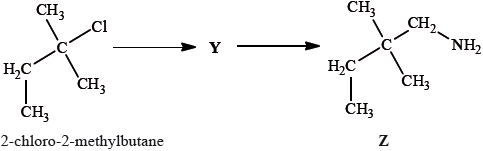
2-chloro-2-methylbutane can also be converted into compound Z by a two-stage reaction via compound Y:
State the other substances required to convert 2-methylbut-2-ene to 2-methylbutan-2-ol.
Explain whether you would expect 2-methylbutan-2-ol to react with acidified potassium dichromate(VI).
State what is meant by optical activity.
State what optical activity indicates about the structure of the molecule.
Optical activity can be detected using a polarimeter. Explain how this works.
Deduce the structural formula of X.
Explain why 2-methylbut-2-ene is less soluble in water than 2-methylbutan-2-ol.
Explain the mechanism of this reaction using curly arrows to represent the movement of electron pairs.
State the rate expression for this reaction and the units of the rate constant.
Suggest why, for some other halogenoalkanes, this hydrolysis is much more effective in alkaline rather than in neutral conditions.
Outline why there are molecules with different molar masses.
Draw the structure of Y.
State the reagent and any catalyst required for both the formation of Y and the conversion of Y into Z.
Formation of Y:
Conversion of Y into Z:
Markscheme
water/\({{\text{H}}_{\text{2}}}{\text{O}}\);
Accept steam.
(concentrated) sulfuric acid/\({{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_{\text{4}}}\) (catalyst);
Accept phosphoric acid/H3PO4.
Award [2] for HBr and NaOH (two-stage process via the halogenoalkane).
not react;
tertiary alcohol (not easily oxidized);
rotates the plane (of polarization) of plane polarized light;
Accept answers in which one of the “plane”s is missing.
two isomers that are enantiomers/chiral/non-superimposable mirror images;
Accept “contains an asymmetric/chiral carbon” or “contains a carbon bonded to four different groups”.
polarizes light / polarized light source;
light passed through sample;
analyser / second polarizer detects whether plane of polarization rotated;
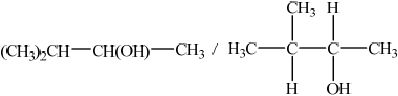 ;
;
Accept C3H7–CH(OH)–CH3, but not CH3–CH2–CH2–CH(OH)–CH3.
2-methylbutan-2-ol has hydroxyl/OH group;
Do not accept “hydroxide group”.
Allow 2-methylbutan-2-ol is an alcohol.
2-methylbutan-2-ol can form H-bonds (to water) / 2-methylbut-2-ene cannot form H-bonds (to water);
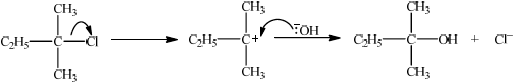
curly arrow showing \({\text{C}}{{\text{l}}^ - }\) leaving;
representation of tertiary carbocation;
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to \({{\text{C}}^ + }\);
Do not allow arrow originating on H in HO–.
formation of organic product \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}{\text{OH}}\) and \({\text{C}}{{\text{l}}^ - }\)/NaCl
(somewhere in mechanism);
Award [3 max] if a candidate gives a fully correct SN2 mechanism.
\({\text{rate}} = {\text{k}} \times \) [2-chloro-2-methylbutane]/\({\text{[C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C(C}}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}{\text{Cl]}}\)/[halogenoalkane]
/[R–Cl];
\({{\text{s}}^{ - 1}}\);
hydroxide ion/\({\text{O}}{{\text{H}}^ - }\) is a better nucleophile than water / hydroxide ion/\({\text{O}}{{\text{H}}^ - }\) has negative charge;
undergo \({{\text{S}}_{\text{N}}}{\text{2}}\) hydrolysis / RDS depends on attack of \({\text{O}}{{\text{H}}^ - }\)/hydroxide ion (nucleophile);
Accept other suggestions that are chemically valid.
chlorine can be \(^{{\text{35}}}{\text{Cl}}\)/Cl–35 or \(^{{\text{37}}}{\text{Cl}}\)/Cl–37;
Accept “chlorine can exist as two isotopes”.
Answer must refer to chlorine rather than isotopes in general.
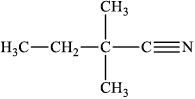 ;
;
Do not accept condensed formulas such as CH3CH2C(CH3)2CN.
Accept the cyanide group as –CN without showing the triple bond.
Formation of Y:
cyanide ion/\({\text{C}}{{\text{N}}^ - }\) / potassium cyanide/KCN;
Accept hydrogen cyanide/HCN.
Conversion of Y into Z:
hydrogen/\({{\text{H}}_{\text{2}}}\);
nickel/Ni / platinum/Pt / palladium/Pd (catalyst);
Examiners report
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.
Many students could recall the reagents for the hydration of an alkene and recognize the alcohol as a tertiary alcohol that would not undergo oxidation. Statements regarding optical activity often lacked precision and betrayed confusion with chirality. Very few could correctly describe how a polarimeter worked, especially the second rotating sheet of polaroid, and students frequently drew the structure of 2-methylbutan-2-ol rather than its chiral isomer. Most students stated that the alcohol was more polar than the alkene, but fewer mentioned that it could form hydrogen bonds to water and even less linked this to the presence of the hydroxyl group. Almost all students recognized that the hydrolysis was \({{\text{S}}_{\text{N}}}{\text{1}}\), with an encouraging number being able to write reasonable mechanisms, though many still lost marks through a lack of precision in where their curly arrows started and ended. Many candidates also stated an appropriate rate equation along with the units of the rate constant. Very few students linked the difference of two molar mass units to the presence in the molecule of chlorine, with its naturally occurring isotopes, and the discussion of any effect on the hydrolysis rate often revealed a lack of clear thinking. In contrast many students correctly identified the nitrile as the intermediate in the chain extension reaction and reagents for its formation and hydrogenation were generally well known.

