| Date | May 2010 | Marks available | 3 | Reference code | 10M.2.hl.TZ1.2 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Determine | Question number | 2 | Adapted from | N/A |
Question
Alex and Hannah were asked to investigate the kinetics involved in the iodination of propanone. They were given the following equation by their teacher.
\[{\text{C}}{{\text{H}}_3}{\text{COC}}{{\text{H}}_3}{\text{(aq)}} + {{\text{I}}_2}{\text{(aq)}}\xrightarrow{{{{\text{H}}^ + }{\text{(aq)}}}}{\text{C}}{{\text{H}}_2}{\text{ICOC}}{{\text{H}}_3}{\text{(aq)}} + {\text{HI(aq)}}\]
Alex’s hypothesis was that the rate will be affected by changing the concentrations of the propanone and the iodine, as the reaction can happen without a catalyst. Hannah’s hypothesis was that as the catalyst is involved in the reaction, the concentrations of the propanone, iodine and the hydrogen ions will all affect the rate.
They carried out several experiments varying the concentration of one of the reactants or the catalyst whilst keeping other concentrations and conditions the same, and obtained the results below.
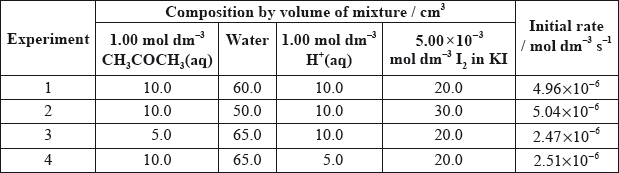
Explain why they added water to the mixtures.
(i) Deduce the order of reaction for each substance and the rate expression from the results.
(ii) Comment on whether Alex’s or Hannah’s hypothesis is correct.
Using the data from Experiment 1, determine the concentration of the substances used and the rate constant for the reaction including its units.
(i) This reaction uses a catalyst. Sketch and annotate the Maxwell-Boltzmann energy distribution curve for a reaction with and without a catalyst on labelled axes below.
(ii) Describe how a catalyst works.
Markscheme
to maintain a constant volume / OWTTE;
(i) \({\text{[}}{{\text{H}}^ + }{\text{]}}\) order 1, \({\text{[C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}{\text{]}}\) order 1, \({\text{[}}{{\text{I}}_{\text{2}}}{\text{]}}\) order 0;
\({\text{(rate}} = {\text{)}}k{\text{[}}{{\text{H}}^ + }{\text{][C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}{\text{]}}\);
Award [2] for correct rate expression.
Allow expressions including [I2]0.
(ii) neither were correct / Alex was right about propanone and wrong about iodine / Hannah was right about propanone and hydrogen ions but wrong about iodine / OWTTE;
\({\text{[C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}{\text{]}} = 0.100{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) and \({\text{[}}{{\text{H}}^ + }{\text{]}} = 0.100{\text{ mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\);
\(k = \frac{{4.96 \times {{10}^{ - 6}}}}{{(0.100 \times 0.100)}} = 4.96 \times {10^{ - 4}}\);
\({\text{mo}}{{\text{l}}^{ - 1}}{\text{d}}{{\text{m}}^{\text{3}}}{{\text{s}}^{ - 1}}\);
Ignore calculation of [I2].
No ECF here for incorrect units.
(i) 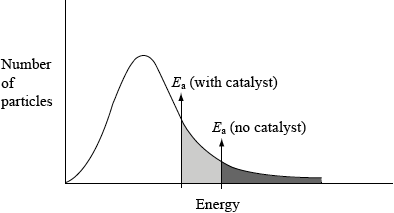
axes correctly labelled x = energy/velocity/speed, y = number/% of molecules/particles;
graph showing correct curve for Maxwell-Boltzmann distribution;
If two curves are drawn, first and second mark can still be scored, but not third.
Curve(s) must begin at origin and not go up at high energy.
two activation energies shown with \({E_{{\text{cat}}}}\) shown lower;
Award the mark for the final point if shown on an enthalpy level diagram.
(ii) catalyst provides an alternative pathway of lower energy / OWTTE;
Accept catalyst lowers activation energy (of reaction).
Examiners report
The presented data in the question proved to be quite tricky for many candidates, and answers to this question were generally disappointing. Very few stated the need to maintain a constant volume in (a) and many thought that water was added in order to provide a solvent for the reagents.
In (b)(i), although the question clearly told candidates to deduce the order for each substance, several did this for only two substances, often the species shown as reactants in the supplied equation. Then the orders shown in the rate expression did not always match the ones deduced. Only the better candidates got the rate expression correct and lots of guess work was seen here. A number gave \({K_c}\) instead of \(k\). The hypothesis question was also poorly answered and many candidates were not prepared for a question where both were incorrect.
Part (c) proved difficult and only the very best candidates got the two concentrations correct most just substituted volumes into their rate expression.
In (d), many candidates drew an enthalpy level diagram and not the Maxwell-Boltzmann distribution curve and others showed two curves. Those that did draw a correct curve often mislabelled the axes. However, the vast majority could explain how a catalyst worked.

