| Date | May 2015 | Marks available | 2 | Reference code | 15M.2.hl.TZ1.7 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | Determine | Question number | 7 | Adapted from | N/A |
Question
Ethanol is a primary alcohol that can be oxidized by acidified potassium dichromate(VI). Distinguish between the reaction conditions needed to produce ethanal and ethanoic acid.
Ethanal:
Ethanoic acid:
Determine the oxidation number of carbon in ethanol and ethanal.
Ethanol:
Ethanal:
Deduce the half-equation for the oxidation of ethanol to ethanal.
Deduce the overall redox equation for the reaction of ethanol to ethanal with acidified potassium dichromate(VI).
Ethanol can be made by reacting aqueous sodium hydroxide with bromoethane.
Explain the mechanism for this reaction, using curly arrows to represent the movement of electron pairs.
Determine the orders of reaction of the reactants and the overall rate expression for the reaction between 2-bromobutane and aqueous sodium hydroxide using the data in the table.
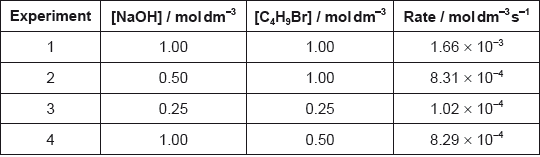
Determine the rate constant, \(k\), with its units, using the data from experiment 3.
Identify the molecularity of the rate-determining step in this reaction.
2-bromobutane exists as optical isomers.
State the essential feature of optical isomers.
2-bromobutane exists as optical isomers.
Outline how a polarimeter can distinguish between these isomers.
Describe the formation of \(\sigma \) and \(\pi \) bonds in an alkene.
The two most abundant isotopes of bromine have the mass numbers 79 and 81.
Calculate the relative abundance of \(^{{\text{79}}}{\text{Br}}\) using table 5 of the data booklet, assuming the abundance of the other isotopes is negligible.
Markscheme
Ethanal: distill off product as it forms;
Accept distillation.
Ethanoic acid: (heat under) reflux / use excess oxidizing agent;
Ethanol: –2/–II;
Ethanal: –1/–I;
Do not accept 2–, 1– but penalize once only.
\({\text{C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH}} \to {\text{C}}{{\text{H}}_3}{\text{CHO}} + {\text{2}}{{\text{H}}^ + } + {\text{2}}{{\text{e}}^ - }\);
Half-equation required. Do not accept \({C_2}{H_5}OH + 2[O] \to C{H_3}CHO + {H_2}O\).
Accept e for \({e^ - }\).
\({\text{3C}}{{\text{H}}_3}{\text{C}}{{\text{H}}_2}{\text{OH(aq)}} + {\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{2C}}{{\text{r}}^{{\text{3}} + }}{\text{(aq)}} + {\text{3C}}{{\text{H}}_3}{\text{CHO(l)}} + {\text{7}}{{\text{H}}_2}{\text{O(l)}}\)
correct reactants and products;
correct balancing;
M2 can only be scored if M1 correct.
Ignore state symbols.
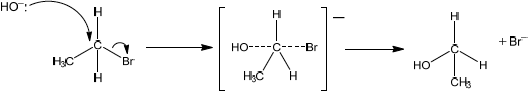
curly arrow going from lone pair/negative charge on O in \({\text{H}}{{\text{O}}^ - }\) to C;
Do not allow curly arrow originating on H in \(H{O^ - }\).
curly arrow showing Br leaving;
Accept curly arrow either going from bond between C and Br to Br in bromoethane or in the transition state.
representation of transition state showing negative charge, square brackets and partial bonds;
Do not penalize if HO and Br are not at 180° to each other.
Do not award M3 if OH----C bond is represented.
formation of organic product \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\) and \({\text{B}}{{\text{r}}^ - }\);
Award [3 max] for correct \({S_N}1\) mechanism.
\({\text{[NaOH] / [O}}{{\text{H}}^ - }{\text{]}}\) is 1/first order and \({\text{[}}{{\text{C}}_4}{{\text{H}}_9}{\text{Br]}}\) is 1/first order;
\({\text{rate }} = k{\text{[O}}{{\text{H}}^ - }{\text{][}}{{\text{C}}_4}{{\text{H}}_9}{\text{Br] / rate}} = k{\text{[NaOH][}}{{\text{C}}_4}{{\text{H}}_9}{\text{Br]}}\);
Square brackets must be used for M2.
\(\left( {\frac{{1.02 \times {{10}^{ - 4}}}}{{0.25 \times 0.25}} = } \right)0.0016/1.6 \times {10^{ - 3}}\);
\({\text{mo}}{{\text{l}}^{ - 1}}\,{\text{d}}{{\text{m}}^{\text{3}}}\,{{\text{s}}^{ - 1}}\);
Accept \({M^{ - 1}} {s^{ - 1}}\).
Ignore order of units.
Must use experiment 3 data.
bimolecular/2;
Accept dimolecular.
chiral/asymmetric carbon / carbon attached to 4 different groups / non-super imposable mirror images;
enantiomers rotate plane of (plane-) polarized light;
in opposite directions (by equal amounts);
Sigma bonds:
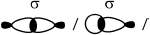
result from head-on/end-on overlap of orbitals / OWTTE;
Accept axial overlap of orbitals.
Accept “symmetric orbital” with respect to same plane / OWTTE.
Pi bonds:
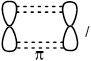
result from sideways overlap of orbitals / OWTTE;
Accept “antisymmetric orbitals” with respect to (defining) plane (containing at least one atom) / OWTTE.
\(79.91 = 79x + 81(1 - x)\);
Award M1 for any suitable calculation.
(abundance \(^{{\text{79}}}{\text{Br}} = \)) 54.5%;
Award [2] for correct final answer.
Examiners report
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.
The idea of “reflux” was usually given for the production of ethanoic acid in (a) but ethanal was less clear. We accept that perhaps we should have phrased (a) (ii), “Determine the average oxidation number of carbon in …” In practice, this was one of the best answered parts and caused few difficulties. Few had any idea how to attempt the half-equation in (iii) and the overall equation in (iv). Although the mechanism in (b) has been set on numerous occasions, candidates are still not taking care over the start and finish of the curly arrows and the intermediate is drawn poorly. It must have partial bonds and the sign must be outside the square brackets. Some candidates offered an \({{\text{S}}_{\text{N}}}{\text{1}}\) mechanism. In (c) (ii), the orders were usually successfully deduced but many omitted to give the overall rate expression. In part (ii), quite a number of candidates unaccountably ignored the instruction and used any experiment but No 3. The units were frequently wrong or omitted. The molecularity was answered satisfactorily. In (d), candidates frequently stated that the molecules have mirror images but not that these mirror images are non-superposable. “Chiral” was a popular correct answer. There seemed to be little understanding of a polarimeter with some suggesting that the crystals themselves rotate. In (e) the equations were poor and few were able to identify the reagent. Most descriptions in (f) would have been improved with a careful and clear diagram. Part (g), the relative abundance of \(^{{\text{79}}}{\text{Br}}\) was well done except by those who tried to do it “by inspection”; this usually led to the wrong answer.

