Half Equations
- Oxidation numbers can be used to balance chemical equations
- Go through these steps to balance a redox equation:
- Write the unbalanced equation and identify the atoms which change in ox. no.
- Deduce the ox.no. changes
- Balance the ox.no. changes
- Balance the charges
- Balance the atoms
Worked Example
Manganate(VII) ions (MnO4-) react with Fe2+ ions in the presence of acid (H+) to form Mn2+ ions, Fe3+ ions and waterWrite the overall redox equation for the reaction
Answer:
Step 1: Write the unbalanced equation and identify the atoms which change in ox. no.

Step 2: Deduce the ox.no. changes
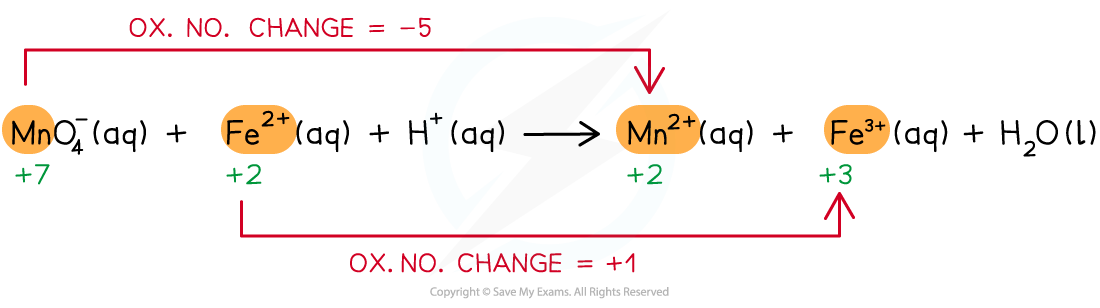
Step 3: Balance the ox.no. changes
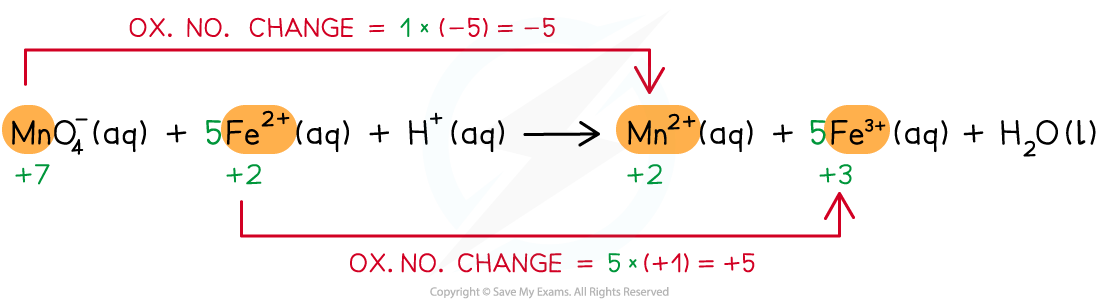
Step 4: Balance the charges
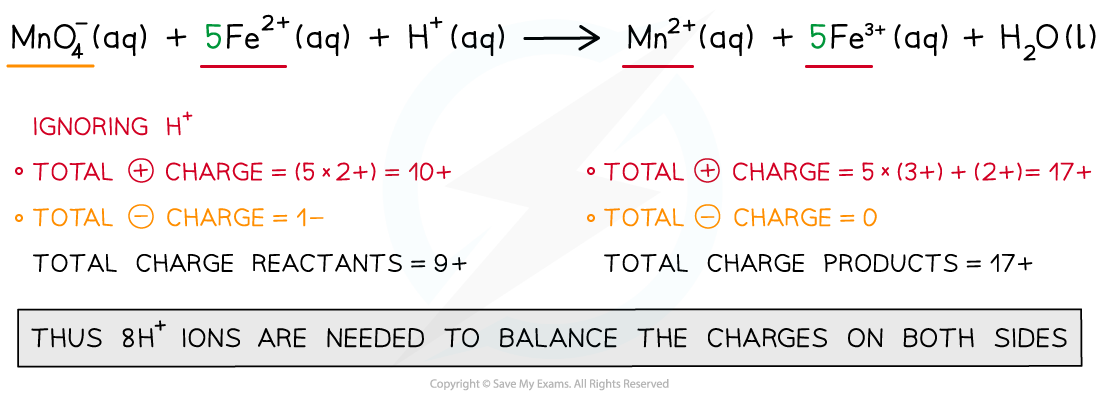
Step 5: Balance the atoms

