How IR Spectroscopy works
- All covalent bonds act rather like springs, as opposed to rigid bars
- Like springs, the bonds can vibrate in a number of different ways
- The frequency of vibration occurs in the infra-red region of the electromagnetic spectrum
- If an organic molecule is irradiated with infra-red energy that matches the natural vibration frequency of its bonds, it absorbs some of that energy and the amplitude of vibration increases
- This is known as resonance

Different modes of vibration in molecules. Each mode has a characteristic frequency of vibration
Infrared (IR) spectroscopy
- Infrared (IR) spectroscopy is a technique used to identify compounds based on changes in vibrations of atoms when they absorb IR of certain frequencies
- A spectrophotometer irradiates the sample with IR radiation and then detects the intensity of IR radiation absorbed by the molecule
- IR energy is absorbed only if a molecule has a permanent dipole that changes as it vibrates
- Symmetrical molecules such as O2 or H2, are therefore IR inactive
- The resonance frequency is the specific frequency at which the bonds will vibrate
- Rather than displaying frequency, an IR spectrum shows a unit called wavenumber
- Wavenumber is the reciprocal of the wavelength and has units of cm-1
- Characteristic absorptions can be matched to specific bonds in molecules
- This enables chemists to determine the functional groups present
Absorption Range of Bonds
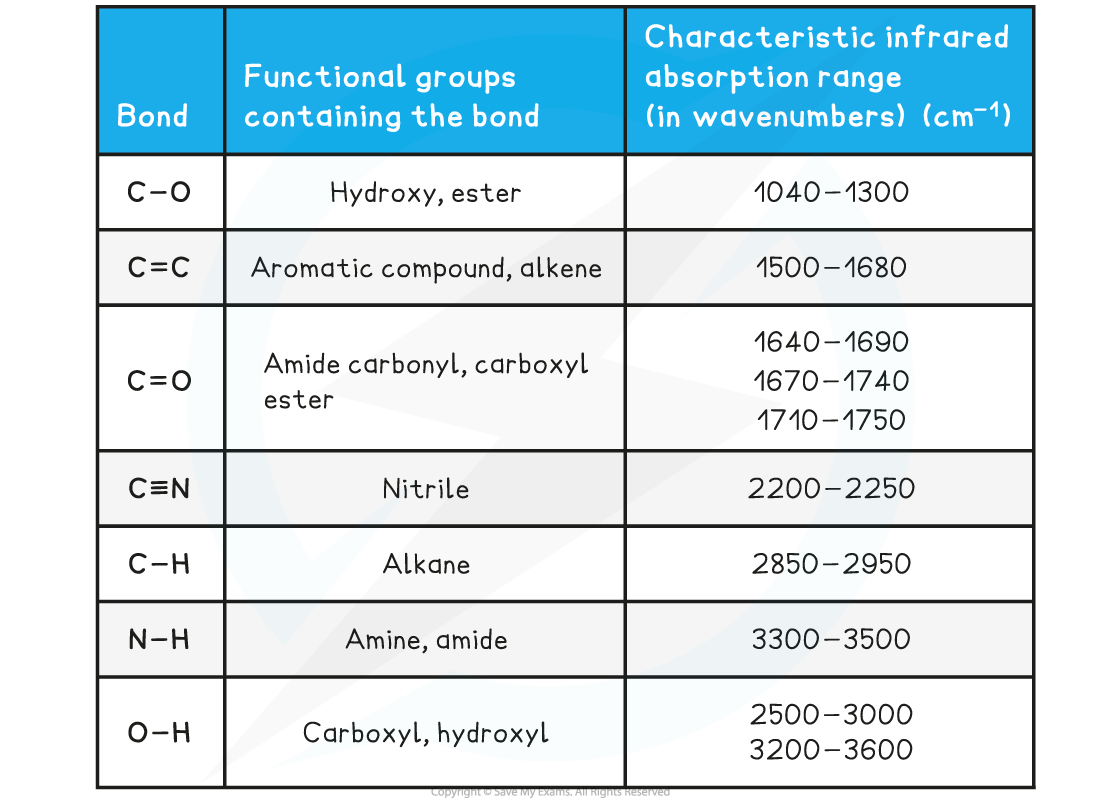
- Due to some absorption bands overlapping each other, other analytical techniques such as mass spectroscopy should be used alongside IR spectroscopy to identify an unknown compound
Interpreting an IR Spectrum
- The best way to understand how to interpret an IR spectrum is by looking at examples and becoming familiar with the characteristic features of an IR spectrum
Worked Example
Examine the two spectra shown and determine which one belongs to propan-2-ol and which one belongs to propanone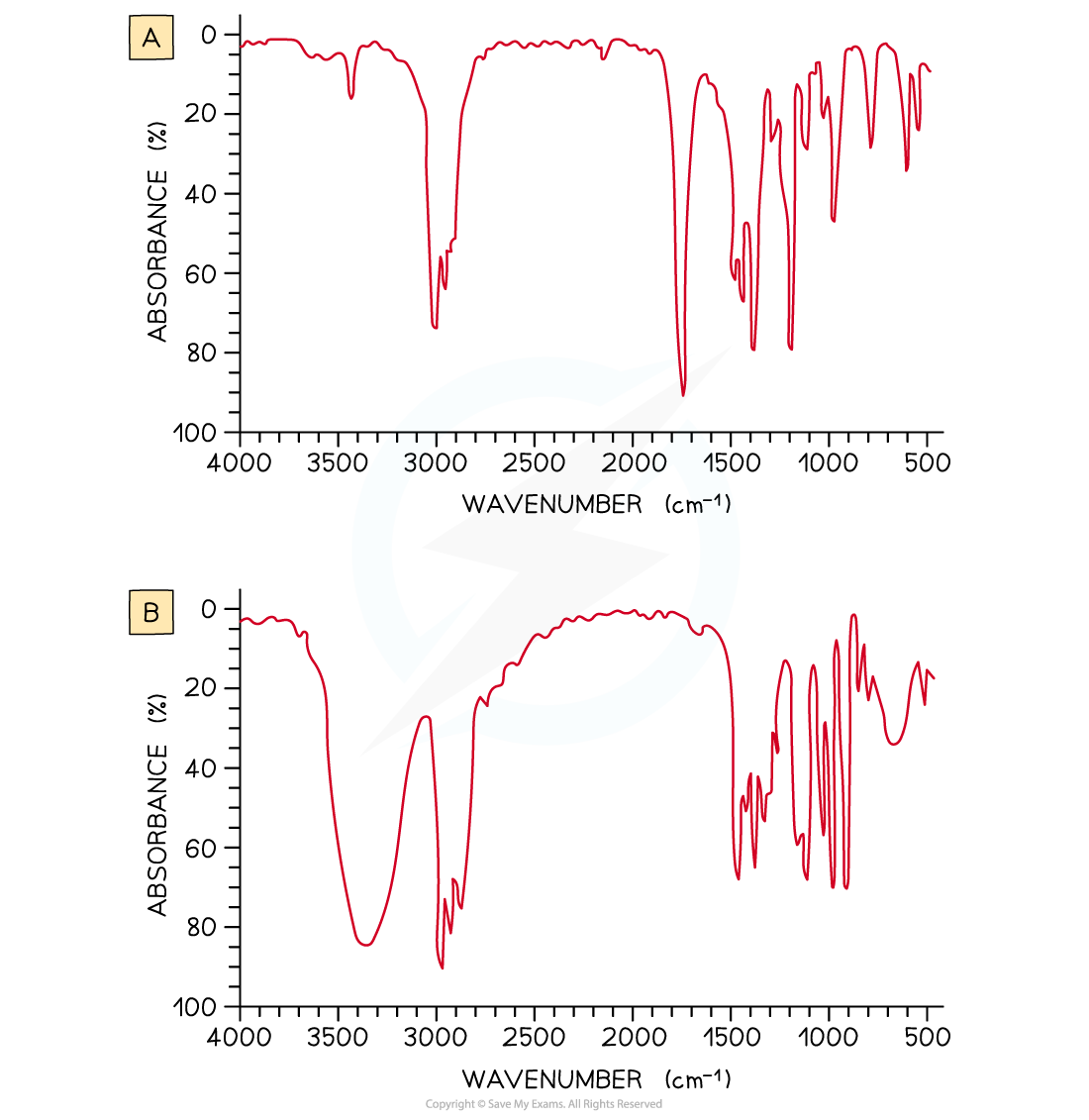
Answer:
- IR spectrum A is propanone and spectrum B is propan-2-ol.
- In IR spectrum A the presence of a strong, sharp absorption around 1710 cm-1 corresponds to the characteristic C=O, carbonyl, group in a ketone.
- In spectrum B the presence of a strong, broad absorption around 3200-3500 cm-1 suggests that there is an alcohol group present, which corresponds to the -OH group in propan-2-ol.
Fingerprint Region
- The region below about 1500 cm-1 is called the fingerprint region and is unique to every molecule
- It has many peaks that can be difficult to assign
- These peaks represent the complex vibrational interactions that occur between different bonds within a molecule
- The value of the fingerprint region is in being able to compare the IR spectrum to a known compund from a database and coming up with an exact match
- This is particularly useful, for example, in identifying a specific member of a homologous series
- All members of the series will show the same type of bonds present, but no two molecules will have the same fingerprint region
Exam Tip
Infrared data is found in Section 26 of the IB Chemistry Data Booklet so there is no need to learn specific wavenumber ranges of bonds
