Reactions of Benzene
- Arenes are very stable compounds due to the delocalisation of π electrons in the ring
- This is because the electron density is spread out over the molecule instead of being confined to a small area
- During chemical reactions such as substitution reactions, this delocalised ring is maintained
- Addition reactions however, disrupt the aromatic stabilisation so they are not favoured
Substitution
- Halogenation reactions are examples of electrophilic substitution reactions
- Arenes undergo substitution reactions with chlorine (Cl2) and bromine (Br2) in the presence of anhydrous AlCl3 or AlBr3 catalyst respectively to form halogenoarenes (aryl halides)
- The chlorine or bromine act as an electrophile and replaces a hydrogen atom on the benzene ring
- The catalyst is required for the reaction to take place, due to the stability of the benzene structure
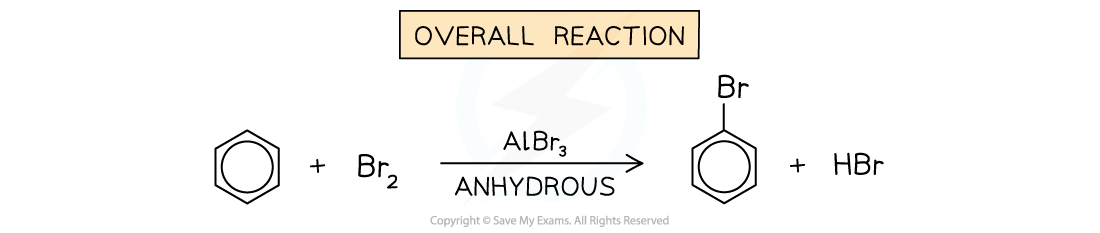
Arenes undergo substitution reactions with halogens to form aryl halides
- Alkylarenes such as methylbenzene undergo halogenation on the 2 or 4 positions
- This is due to the electron-donating alkyl groups which activate these positions
- The halogenation of alkylarenes therefore result in the formation of two products
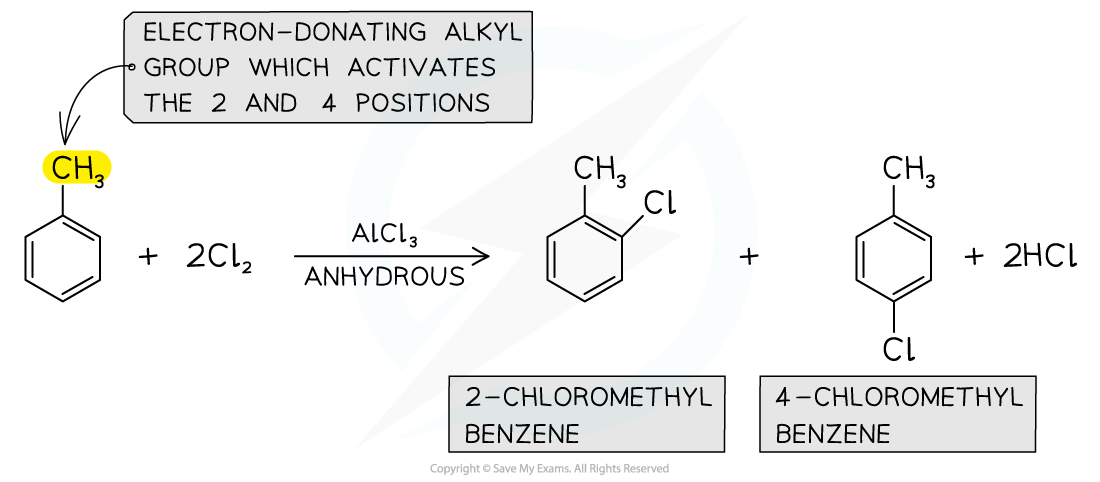
Alkylarenes are substituted on the 2 or 4 position
- Multiple substitutions occur when excess halogen is used
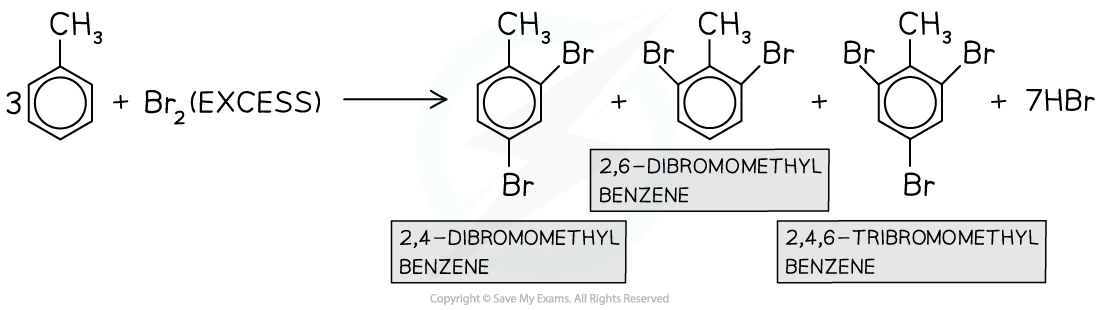
In the presence of excess halogen, multiple substitutions occur
Nitration
- Another example of a substitution reaction is the nitration of arenes
- In these reactions, a nitro (-NO2) group replaces a hydrogen atom on the arene
- The benzene is reacted with a mixture of concentrated nitric acid (HNO3) and concentrated sulfuric acid (H2SO4) at a temperature between 25 and 60 oC

Nitration of benzene
- Again, due to the electron-donating alkyl groups in alkylarenes, nitration of methylbenzene will occur on the 2 and 4 position
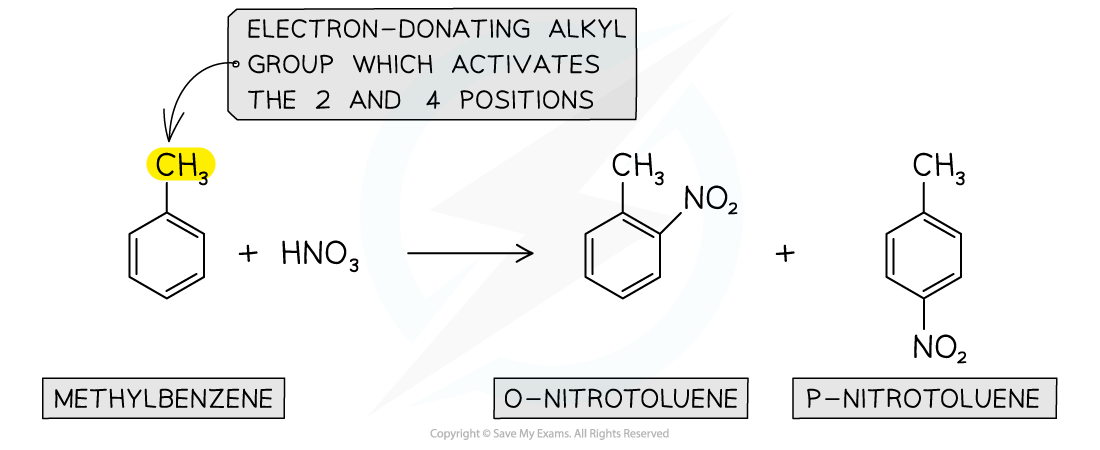
Nitration of alkylarenes
