Halogenation of Alkenes
- The reaction between alkenes and halogens is known as halogenation
- It is an example of an electrophilic addition where an electrophile ('electron seeker') joins onto to a double bond
- The C=C double bond is broken, and a new single bond is formed from each of the two carbon atoms
- The result of this reaction is a dihalogenoalkane
- The reaction occurs readily at room temperature and is the basis for the test for unsaturation in molecules

Halogenation in alkenes
- Halogens can be used to test if a molecule is unsaturated (i.e. contain a double bond)
- Br2 is an orange or yellow solution, called bromine water
- The unknown compound is shaken with the bromine water
- If the compound is unsaturated, an addition reaction will take place and the coloured solution will decolourise
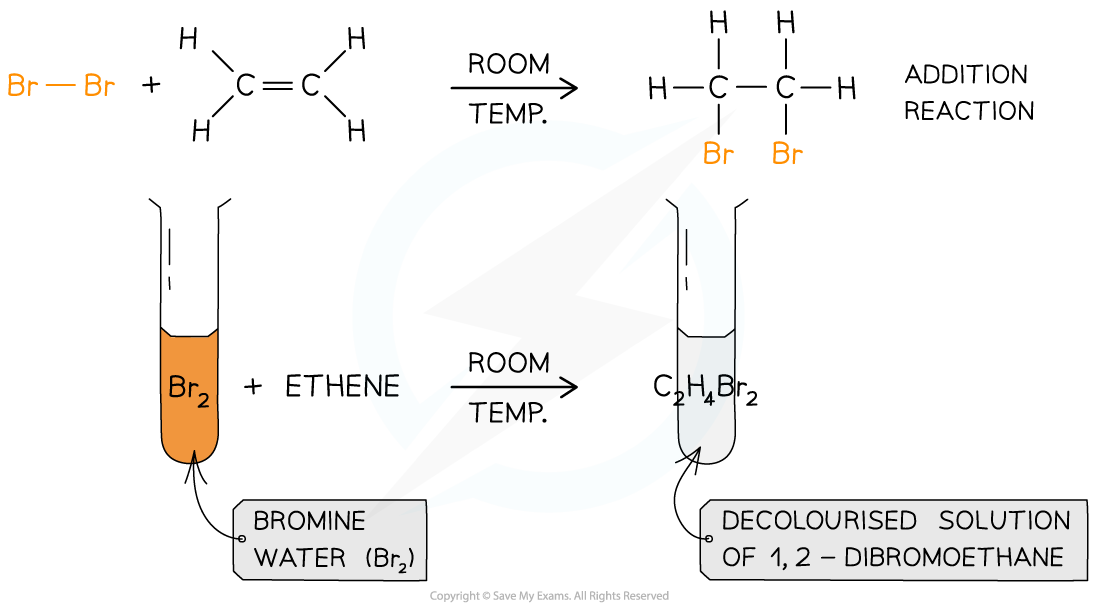
The bromine water test is the standard test for unsaturation in alkenes
Exam Tip
The mechanism of this reaction is part of Higher Level Chemistry and is covered in Section 20
