First Ionisation Energy
- The ionisation energy (IE) of an element is the amount of energy required to remove one mole of electrons from one mole of atoms of an element in the gaseous state to form one mole of gaseous ions
- Ionisation energies are measured under standard conditions which are 298 K and 100 kPa
- The units of IE are kilojoules per mole (kJ mol-1)
- The first ionisation energy is the energy required to remove the one mole of electrons from one mole the gaseous atoms
- E.g. the first ionisation energy of calcium:
Ca(g) → Ca+ (g) + e- 1st ∆H I.E. = +590 kJ mol-1
Ionisation Energies: Trends
- Ionisation energies show periodicity
- As could be expected from their electronic configuration, the group I metals show low IE whereas the noble gases have very high IEs
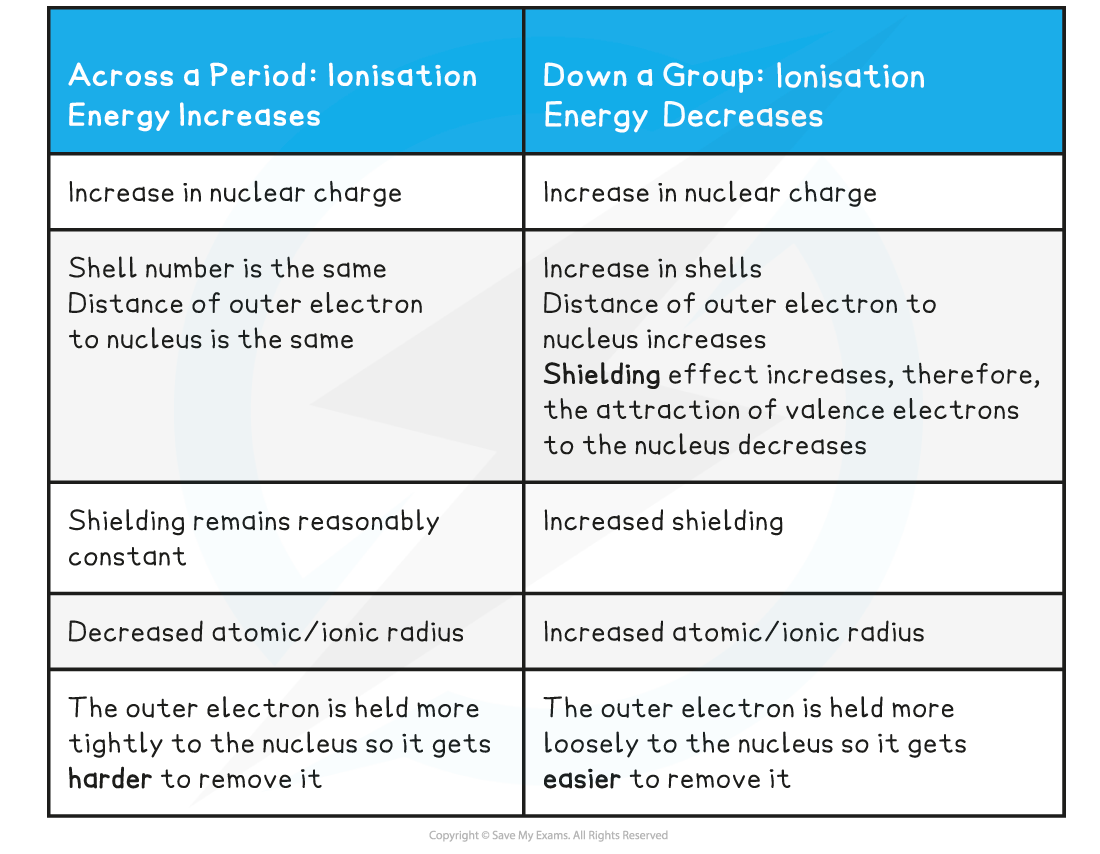
- The first ionisation energy increases across a period and decreases down a group and is caused by four factors that influence the ionisation energy:
- Size of the nuclear charge: the nuclear charge increases with increasing atomic number, which means that there are greater attractive forces between the nucleus and outer electrons, so more energy is required to overcome these attractive forces when removing an electron
- Distance of outer electrons from the nucleus: electrons in shells that are further away from the nucleus are less attracted to the nucleus so the further the outer electron shell is from the nucleus, the lower the ionisation energy
- Shielding effect of inner electrons: the shielding effect is when the electrons in full inner shells repel electrons in outer shells preventing them to feel the full nuclear charge so the greater the shielding of outer electrons by inner electron shells, the lower the ionisation energy
- Spin-pair repulsion: paired electrons in the same atomic orbital in a subshell repel each other more than electrons in different atomic orbitals; this makes it easier to remove an electron (which is why the first ionization energy is always the lowest)

A graph showing the ionisation energies of the elements hydrogen to sodium
Ionisation energy across a period
- The ionisation energy across a period increases due to the following factors:
- Across a period the nuclear charge increases
- The distance between the nucleus and outer electron remains reasonably constant
- The shielding by inner shell electrons remains the same
- There is a rapid decrease in ionisation energy between the last element in one period and the first element in the next period caused by:
- The increased distance between the nucleus and the outer electrons
- The increased shielding by inner electrons
- These two factors outweigh the increased nuclear charge
- There is a slight decrease in 1st I.E. between beryllium and boron as the fifth electron in boron is in the 2p subshell which is further away from the nucleus than the 2s subshell of beryllium
- Beryllium has a first ionisation energy of 900 kJ mol-1 as its electron configuration is 1s2 2s2
- Boron has a first ionisation energy of 801 kJ mol-1 as its electron configuration is 1s2 2s2 2p1
- There is a slight decrease in 1st I.E. between nitrogen and oxygen due to spin-pair repulsion in the 2p subshell of oxygen
- Nitrogen has a first ionisation energy of 1402 kJ mol-1 as its electron configuration is 1s2 2s2 2p3
- Oxygen has a first ionisation energy of 1314 kJ mol-1 as its electron configuration is 1s2 2s2 2p4
Ionisation energy down a group
- Although going down a group the nuclear charge increases, the ionisation energy down a group decreases and it is due to the following factors:
- The distance between the nucleus and outer electron increases
- The shielding by inner shell electrons increases
- The effective nuclear charge is decreasing as shielding increases
Ionisation Energy Trends across a Period & going down a Group Table
Successive ionisation energies of an element
- The successive ionisation energies of an element increase as removing an electron from a positive ion is more difficult than from a neutral atom
- As more electrons are removed the attractive forces increase due to decreasing shielding and an increase in the proton to electron ratio
- The increase in ionisation energy, however, is not constant and is dependent on the atom's electronic configuration
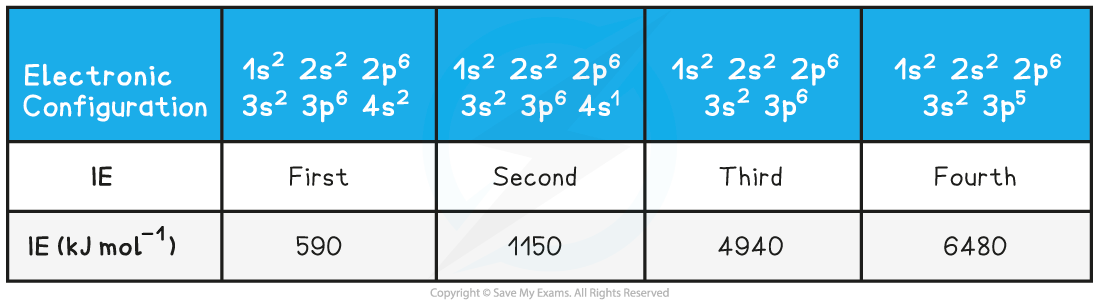
- Taking calcium as an example:
Ionisation Energies of Calcium Table
- The values become very large and difficult to represent meaningfully, so it is more convenient to show the logarithm of the ionisation energies
- This helps us to see significant jumps in I.E.

Successive ionisation energies for the element calcium
- The first electron removed has a low ionisation energy as it is easily removed from the atom due to the spin-pair repulsion of the electrons in the 4s orbital
- The second electron is a little more difficult to remove than the first electron as you are removing an electron from a positively charged ion
- The third electron is much more difficult to remove than the second one corresponding to the fact that the third electron is in a principal quantum shell which is closer to the nucleus (3p)
- The graph shows there is a large increase in successive ionisation energy as the electrons are being removed from an increasingly positive ion
- The big jumps on the graph show the change of shell and the small jumps are the change of subshell
Exam Tip
Be careful with how you interpret successive ionisation energy graphs as it is common for students to read them the wrong way around and count outer electrons from right to left instead of left to right so they get the jumps in the wrong place. This happens particularly when you are given only a partial successive ionisation energy graph and have to deduce which group the element comes from.It's a good idea if you see an ionisation energy graph in an exam question to label the shells and subshells so you are less likely to make this mistake!
