| Date | May 2009 | Marks available | 4 | Reference code | 09M.2.sl.TZ2.7 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Deduce, Draw, Explain, and State | Question number | 7 | Adapted from | N/A |
Question
Define the term isotopes.
A sample of silicon contains three isotopes.
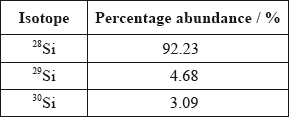
Calculate the relative atomic mass of silicon using this data.
Describe the structure and bonding in silicon dioxide and carbon dioxide.
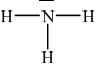
Draw the Lewis structure of NH3, state its shape and deduce and explain the H–N–H bond angle in \({\text{N}}{{\text{H}}_{\text{3}}}\).
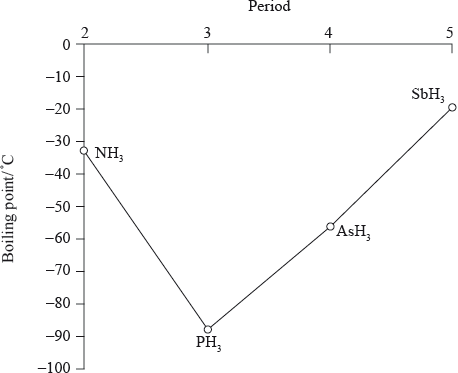
The graph below shows the boiling points of the hydrides of group 5. Discuss the variation in the boiling points.
Explain, using diagrams, why CO and \({\text{N}}{{\text{O}}_{\text{2}}}\) are polar molecules but \({\text{C}}{{\text{O}}_{\text{2}}}\) is a non-polar molecule.
Markscheme
atoms of the same element with the same atomic number/Z/same number of protons, but different mass numbers/A/different number of neutrons;
\((0.9223 \times 28) + (0.0468 \times 29) + (0.0309 \times 30)\);
28.1/28.11;
Working must be shown to get [2], do not accept 28.09 on its own (given in the data booklet).
Silicon dioxide
single covalent (bonds);
network/giant covalent/ macromolecular / repeating tetrahedral units;
Carbon dioxide
double covalent (bonds);
(simple / discrete) molecular;
Marks may be obtained from suitable structural representations of SiO2 and CO2.
 ;
;
Allow crosses or dots for lone-pair.
trigonal/triangular pyramidal;
(\( \sim \))107° / less than 109.5°;
Do not allow ECF.
LP-BP repulsion \( > \) BP-BP repulsion / one lone pair and three bond pairs / lone pairs/non-bonding pairs repel more than bonding-pairs;
Do not accept repulsion between atoms.
boiling points increase going down the group (from \({\text{P}}{{\text{H}}_{\text{3}}}\) to \({\text{As}}{{\text{H}}_{\text{3}}}\) to \({\text{Sb}}{{\text{H}}_{\text{3}}}\));
\({M_{\text{r}}}\)/number of electrons/molecular size increases down the group;
Accept electron cloud increases down the group for the second marking point.
greater dispersion/London/van der Waal’s forces;
\({\text{N}}{{\text{H}}_{\text{3}}}\)/ammonia has a higher boiling point than expected due to the hydrogen bonding between the molecules;
Do not accept hydrogen bonding alone.
CO:
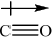
Award [1] for showing the net dipole moment, or explaining it in words (unsymmetrical distribution of charge).
\(N{O_2}\):
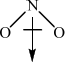
Award [1] for correct representation of the bent shape and [1] for showing the net dipole moment, or explaining it in words (unsymmetrical distribution of charge).
\(C{O_2}\):
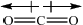
Award [1] for correct representation of the linear shape and [1] for showing the two equal but opposite dipoles or explaining it in words (symmetrical distribution of charge).
For all three molecules, allow either arrow or arrow with bar for representation of dipole moment.
Allow correct partial charges instead of the representation of the vector dipole moment.
Ignore incorrect bonds.
Lone pairs not needed.
Examiners report
In general the definition of isotopes was correct in (a) (i), but there are still some candidates who stated “isotopes are elements” and not “atoms of the same element”.
Nearly everybody gave the correct answer of 28.1 for the relative atomic mass of silicon in (ii).
Part (a) (iii) proved to be very difficult for the candidates. There was a lot of confusion about the two molecules; some candidates stated that they had the same double bond. Not many candidates mentioned the giant covalent structure for the silicon dioxide or the simple molecular structure for the carbon dioxide.
In (b) (i) the majority of candidates drew the Lewis structure of the ammonia molecule correctly showing the lone pair of electrons and the correct shape and angle and (ii) was well answered by most candidates.
They realised that \({\text{N}}{{\text{H}}_{\text{3}}}\) had a higher boiling point than \({\text{P}}{{\text{H}}_{\text{3}}}\) because of the intermolecular hydrogen bonding present in \({\text{N}}{{\text{H}}_{\text{3}}}\).
For (c) most answers given here showed diagrams of the three molecules, including distribution of charges, bonding and shapes. Some candidates gave very good answers showing a good understanding of the polarity of molecules.

