| Date | November 2014 | Marks available | 1 | Reference code | 14N.2.hl.TZ0.5 |
| Level | HL | Paper | 2 | Time zone | TZ0 |
| Command term | Identify | Question number | 5 | Adapted from | N/A |
Question
Graphite has a layered structure of carbon atoms. A section of the structure is shown below.
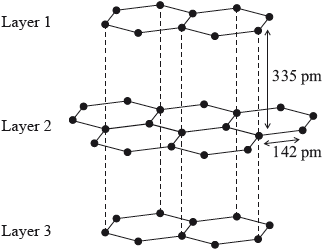
Identify the type of attraction represented by the dotted lines shown between the layers.
Graphite is used as a lubricant. Discuss two other uses of graphite with reference to its layered structure.
Markscheme
van der Waals’/vdW/London/dispersion (forces)/LDF / temporary/instantaneous/induced dipoles;
Two of the following pairs:
used as pencil (lead);
layers can flake off/slide off/break off/stick to paper / OWTTE;
M2 must contain concept of separation of layers, so do not award mark for phrases like "layers can slide over each other" on their own.
OR
used as carbon fibre / OWTTE;
bonding within layer is strong / layers are extensive / layers are strong;
OR
used as electrodes/conductor/in batteries;
has mobile/free/delocalized electrons (between layers) / electricity flows parallel to layers;
OR
used for thermal insulation;
vibrations are not easily passed between layers;
Accept other valid uses of graphite along with a suitable explanation.
Examiners report
Less than half the candidates recognized van der Waals’ forces between the layers in graphite. Some candidates identified the type of attraction as “electrostatic” and others as “intermolecular forces” which were too general and did not score the mark.
Well answered generally. Most candidates gave two uses (usually pencil lead and electrical conductor) and they were often able to explain the uses in terms of the structure.
Syllabus sections
- 17N.2.sl.TZ0.3b: Predict with a reason, whether the molecule PF3 is polar or non-polar.
- 17N.2.sl.TZ0.3a: Draw the Lewis (electron dot) structures of PF3 and PF4+ and use the VSEPR theory to deduce...
- 17N.2.hl.TZ0.4b: Predict whether the molecules PF3 and PF5 are polar or non-polar.
- 17N.2.hl.TZ0.4a: Draw the Lewis (electron dot) structures of PF3 and PF5 and use the VSEPR theory to deduce...
- 17M.2.hl.TZ1.5c: Ammonia reacts reversibly with...
- 17M.2.hl.TZ1.5a: Estimate the H−N−H bond angle in methanamine using VSEPR theory.
- 17M.2.hl.TZ2.4b.ii: Deduce one resonance structure of ozone and the corresponding formal charges on each oxygen...
- 17M.2.sl.TZ2.4b: Deduce the Lewis (electron dot) structures of ozone.
- 17M.2.sl.TZ2.3b: Deduce the Lewis (electron dot) structure and molecular geometry of PCl3.
- 17M.1.sl.TZ2.11: What are the approximate bond angles and structure of crystalline SiO2?
- 17M.2.hl.TZ1.5b: State the electron domain geometry around the nitrogen atom and its hybridization...
- 17M.2.sl.TZ1.4a: Estimate the H−N−H bond angle in methanamine using VSEPR theory.
- 17M.1.hl.TZ1.12: Which combination describes the bonding and structure in benzoic acid, C6H5COOH?
- 17M.1.sl.TZ1.12: Which correctly states the strongest intermolecular forces in the compounds below?
- 17M.1.sl.TZ1.11: Which combination describes the sulfate(IV) ion, SO32– (also known as sulfite ion)?
- 17M.1.sl.TZ1.9: A substance has the following properties: What is the most probable structure of this...
- 16N.3.sl.TZ0.6c: (i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in...
- 16N.2.hl.TZ0.2e: Outline why all the C–O bond lengths in the ethanedioate ion are the same length and suggest...
- 16N.2.hl.TZ0.2d: Draw the Lewis (electron dot) structure of the ethanedioate ion, –OOCCOO–.
- 16N.2.sl.TZ0.2d: The Lewis (electron dot) structure of the ethanedioate ion is shown below. Outline why all...
- 16N.1.sl.TZ0.12: Which substance has a giant covalent structure?
- 16N.1.sl.TZ0.9: Which pair of molecules has the same bond angles? A. PCl3 and BCl3 B. SO2 and CO2 C....
- 16M.2.hl.TZ0.3c: One of the intermediates in the reaction between nitrogen monoxide and hydrogen is dinitrogen...
- 16M.2.hl.TZ0.1a: (i) Draw a Lewis (electron dot) structure of phosphine. (ii) State the hybridization of the...
- 16M.2.sl.TZ0.1b: Phosphine is usually prepared by heating white phosphorus, one of the allotropes of...
- 16M.2.sl.TZ0.1a: (i) Draw a Lewis (electron dot) structure of phosphine. (ii) Outline whether you expect the...
- 16M.1.sl.TZ0.11: Which compound has resonance structures? A. C6H12 B. CH3CHO C. NaBr D. Na2CO3
- 15M.1.hl.TZ1.11: Which substance has the following properties? • Low melting point • Very soluble in...
- 15M.1.hl.TZ2.10: Which diagrams can be used to represent the Lewis (electron dot) structure of boron...
- 15M.2.hl.TZ1.1d: Deduce the Lewis (electron dot) structure of ethanedioic acid, \({\text{HOOC–COOH}}\).
- 15M.2.hl.TZ1.2b.ii: Predict whether phosphorus(V) oxide and sodium oxide conduct electricity in their solid and...
- 15M.2.hl.TZ1.2b.i: Explain why the melting point of phosphorus(V) oxide is lower than that of sodium oxide in...
- 15M.2.hl.TZ1.5g: Identify three allotropes of carbon and describe their structures.
- 15M.1.sl.TZ1.10: Which molecules react to form a dative covalent (coordinate) bond? A. ...
- 15M.1.sl.TZ1.11: What describes the relationship between diamond, graphite and \({{\text{C}}_{{\text{60}}}}\)...
- 15M.1.sl.TZ1.9: What describes the structure of silicon and silicon dioxide?
- 15M.1.sl.TZ2.10: Which species contain a dative covalent (coordination or coordinate) bond? I. Carbon...
- 15M.1.sl.TZ2.11: Which combination of shape and bond angle best describes a molecule of sulfur dioxide,...
- 15M.2.sl.TZ1.1e: Deduce the Lewis (electron dot) structure of ethanedioic acid, HOOC−COOH.
- 15M.2.sl.TZ1.6g: Covalent bonds form when phosphorus reacts with chlorine to form...
- 15M.2.sl.TZ2.6a.i: Draw the Lewis (electron dot) structure of chloromethane.
- 15M.2.sl.TZ2.6a.ii: Predict the shape of the chloromethane molecule and the H–C–H bond...
- 15M.2.sl.TZ2.6a.iii: Explain why chloromethane is a polar molecule.
- 14M.1.hl.TZ1.11: A solid has a melting point of 1582 °C and does not dissolve in water. It does not conduct...
- 14M.2.hl.TZ1.1d: Magnesium sulfate is one of the products formed when acid rain reacts with dolomitic...
- 14M.2.hl.TZ1.5a: (i) State the changes in the acid-base nature of the oxides across period 3 (from...
- 14M.2.hl.TZ2.4b: State the shape of the ozone molecule and estimate the bond angle. Shape: Bond angle:
- 14M.1.sl.TZ1.11: What is the shape and the bond angle of the molecule \({\text{B}}{{\text{F}}_{\text{3}}}\)?
- 14M.1.sl.TZ2.12: Which pair has the same bond angles? A. ...
- 14M.2.sl.TZ1.1e: (i) State the equation for the reaction of sulfuric acid with magnesium...
- 14M.1.sl.TZ2.13: Which diagram represents the bonding in \({\text{Si}}{{\text{O}}_{\text{2}}}\)?
- 14M.2.sl.TZ2.4c.v: Draw the Lewis (electron dot) structure of chloric(I) acid.
- 14M.2.sl.TZ2.4c.vi: Predict the H–O–Cl bond angle in this molecule and explain this in terms of the valence shell...
- 14M.3.sl.TZ1.24d: State the formula and deduce the shape of the positive ion (cation) formed when...
- 14N.1.hl.TZ0.13: Which group of ions and molecules has delocalized electrons in all the species? A. ...
- 14N.2.hl.TZ0.5b: Graphite is used as a lubricant. Discuss two other uses of graphite with reference to its...
- 14N.1.sl.TZ0.13: Which species contains a bond angle of approximately 107°? A. ...
- 14N.1.sl.TZ0.9: Which species contains a dative covalent (coordinate) bond? A. HCN B. ...
- 14N.2.sl.TZ0.3a: Explain why the distance between adjacent carbon atoms within a layer is shorter than the...
- 14N.2.sl.TZ0.3b: Graphite is used as a lubricant. Discuss two other uses of graphite with reference to its...
- 14N.2.sl.TZ0.6e: (i) Deduce the Lewis structure of \({\text{PH}}_4^ + \). (ii) Predict, giving a...
- 13N.2.hl.TZ0.4b: State the name given to species that bond to a central metal ion, and identify the type of...
- 13N.2.hl.TZ0.5h: Deduce the N–N–N bond angle in trinitramide and explain your reasoning.
- 13N.2.hl.TZ0.5i: Predict, with an explanation, the polarity of the trinitramide molecule.
- 13N.2.hl.TZ0.6e.i: Compare the properties of the three oxides by completing the table below.
- 13N.2.hl.TZ0.6e.iii: As well as the oxide above, sodium forms a peroxide that contains the peroxide ion,...
- 13N.1.sl.TZ0.12: The Lewis (electron dot) structure of aspirin is represented below. What are the...
- 13N.2.sl.TZ0.4e: Deduce the N–N–N bond angle in trinitramide and explain your reasoning.
- 13N.2.sl.TZ0.4f: Predict, with an explanation, the polarity of the trinitramide molecule.
- 13M.2.hl.TZ1.6c.v: Compare the melting points of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and...
- 13M.1.sl.TZ1.13: Which combination best describes the type of bonding present and the melting point of silicon...
- 13M.2.sl.TZ1.6c.i: Deduce the Lewis structures of \({\text{N}}{{\text{H}}_{\text{3}}}\) and...
- 13M.2.sl.TZ1.6c.iii: Compare the shapes of the two molecules and explain the difference using valence shell...
- 13M.2.sl.TZ1.6c.iv: Predict and explain whether the molecules \({\text{N}}{{\text{H}}_{\text{3}}}\) and...
- 13M.2.hl.TZ2.3b: State the type of bonding between platinum and nitrogen in carboplatin.
- 13M.2.hl.TZ2.5b.ii: State and explain the Cl–P–Cl bond angle in PCl3.
- 13M.1.sl.TZ2.11: Which statements about graphite are correct? I. Carbon atoms are held in layers with...
- 13M.1.sl.TZ2.13: Which statements about the structure and bonding of silicon dioxide are correct?
- 13M.2.sl.TZ2.5b.i: Deduce the Lewis (electron dot) structure and predict the shape of each molecule, using the...
- 13M.2.sl.TZ2.5b.ii: State and explain the F–S–F bond angle in SF2.
- 13M.2.sl.TZ2.5b.iii: Deduce whether each of the three molecules is polar or non-polar, giving your reason in each...
- 12N.1.sl.TZ0.12: Diamond, C60 fullerene and graphite are allotropes of carbon. Which statements are correct...
- 12N.1.sl.TZ0.13: Which statement about the physical properties of substances is correct? A. The only...
- 12N.2.sl.TZ0.2b: (i) Determine \(\Delta H\), the enthalpy change of the reaction, in...
- 12N.2.sl.TZ0.5b.ii: The Lewis (electron dot) structure of nitrous acid is given below. Identify which...
- 12N.2.sl.TZ0.5b.v: Ammonia, NH3, is a weak base. Deduce the Lewis (electron dot) structure of NH3. State the...
- 12N.2.sl.TZ0.5b.iii: Deduce the approximate value of the hydrogen-oxygen-nitrogen bond angle in nitrous acid and...
- 10N.1.hl.TZ0.12: Which molecule has an octahedral shape? A. SF6 B. PCl5 C. XeF4 D. BF3
- 10N.2.hl.TZ0.7d: The reaction between \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(aq)}}\) and...
- 10N.1.sl.TZ0.11: Which species contain a dative covalent bond? I. HCHO II. CO III. ...
- 10N.2.sl.TZ0.4f: (i) Identify the type of reaction that occurs. (ii) Predict the value of the H–N–H...
- 09N.1.hl.TZ0.11: What is the bond angle in the \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) ion? A. ...
- 09N.1.hl.TZ0.13: How many atoms is each carbon directly bonded to in its allotropes?
- 09N.1.sl.TZ0.11: How many non-bonding pairs of electrons are there in a nitrogen molecule? A. 0 B. ...
- 09N.1.sl.TZ0.12: Which molecule contains a bond angle of approximately 120°? A. ...
- 09N.2.sl.TZ0.2: PF3, SF2 and SiF4 have different shapes. Draw their Lewis structures and use the VSEPR theory...
- 10M.2.sl.TZ1.3a.i: Draw the Lewis structure for chloroethene and predict the H–C–Cl bond angle.
- 10M.1.sl.TZ2.14: Which compound has a covalent macromolecular (giant covalent) structure? A. MgO(s) B. ...
- 10M.1.sl.TZ2.11: What is the shape of the ammonia molecule, \({\text{N}}{{\text{H}}_{\text{3}}}\)? A. ...
- 10M.1.sl.TZ2.12: Which molecule is polar? A. ...
- 10M.2.sl.TZ2.7a.ii: Deduce the Lewis structure of chloroethene and identify the formula of the repeating unit of...
- 09M.1.hl.TZ1.11: Which molecule contains a dative covalent (coordinate) bond? A. HCN B. ...
- 09M.2.hl.TZ1.6c.iii: Deduce all the bond angles present in propene.
- 09M.1.sl.TZ1.14: Which is the best description of the bonding present in silicon dioxide,...
- 09M.2.sl.TZ1.6b.i: \({\text{C}}{{\text{O}}_{\text{2}}}\)
- 09M.2.sl.TZ1.6b.ii: \({\text{CO}}_3^{2 - }\)
- 09M.2.sl.TZ1.6b.iii: \({\text{BF}}_4^ - \)
- 09M.2.hl.TZ2.5b.ii: \({\text{NO}}_2^ + \)
- 09M.2.sl.TZ2.7a.iii: Describe the structure and bonding in silicon dioxide and carbon dioxide.
- 09M.2.sl.TZ2.7b.i: Draw the Lewis structure of NH3, state its shape and deduce and explain the H–N–H bond angle...
- 11M.1.hl.TZ1.14: The Lewis structure of \({\text{S}}{{\text{O}}_{\text{2}}}\) is given below. What is the...
- 11M.2.hl.TZ1.6f.i: Explain the electrical conductivity of molten sodium oxide and liquid sulfur trioxide.
- 11M.1.sl.TZ1.11: How do the bond angles in \({\text{C}}{{\text{H}}_{\text{4}}}\),...
- 11M.1.sl.TZ1.9: What is the correct Lewis structure for hypochlorous acid, a compound containing chlorine,...
- 11M.2.sl.TZ1.5b.ii: sodium oxide has a higher melting point than sulfur trioxide.
- 11M.2.sl.TZ1.7d.i: Draw the Lewis structure of \({\text{C}}{{\text{O}}_{\text{2}}}\) and predict its shape and...
- 11M.2.sl.TZ1.7d.iii: Explain why silicon dioxide is a solid and carbon dioxide is a gas at room temperature.
- 11M.2.sl.TZ1.7d.ii: Describe the structure and bonding in \({\text{Si}}{{\text{O}}_{\text{2}}}\).
- 11M.2.sl.TZ1.7c: Describe and compare three features of the structure and bonding in the three allotropes of...
- 11M.1.sl.TZ2.10: Which molecule has a non-bonding (lone) pair of electrons on the central atom? A. ...
- 11M.1.sl.TZ2.13: Lewis structures are represented in different ways in different parts of the world. Two ways...
- 12M.1.hl.TZ2.9: Which species contain dative covalent bonds? I. CO II. ...
- 12M.1.sl.TZ2.12: The Lewis (electron dot) structure of paracetamol (acetaminophen) is: What are the...
- 12M.1.sl.TZ2.13: \({{\text{C}}_{{\text{60}}}}\) fullerene consists of a simple molecular structure. Silicon...
- 12M.2.sl.TZ2.5c: Graphite is used as a lubricant and is an electrical conductor. Diamond is hard and does not...
- 12M.2.sl.TZ2.6c: (i) When ammonium chloride, \({\text{N}}{{\text{H}}_{\text{4}}}{\text{Cl(aq)}}\), is...
- 11N.2.hl.TZ0.6b.i: State and explain the difference in the electrical conductivity in the liquid state of the...
- 11N.2.hl.TZ0.8e.i: Identify the type of bond present between \({\text{B}}{{\text{F}}_{\text{3}}}\) and...
- 11N.2.sl.TZ0.5c.ii: Explain why \({\text{PB}}{{\text{r}}_{\text{3}}}\) is a polar molecule.
- 11N.1.sl.TZ0.10: Which row correctly describes the bonding type and melting point of carbon and carbon dioxide?
- 11N.1.sl.TZ0.12: Which is the correct Lewis structure for ethene?
- 11N.2.sl.TZ0.1c.ii: The metal oxides from the second reaction then react with silicon dioxide to form a silicate...
- 11N.2.sl.TZ0.5c.i: For each of the species \({\text{PB}}{{\text{r}}_{\text{3}}}\) and HCHO: • deduce the...

