| Date | November 2011 | Marks available | 1 | Reference code | 11N.1.sl.TZ0.10 |
| Level | SL | Paper | 1 | Time zone | TZ0 |
| Command term | Identify | Question number | 10 | Adapted from | N/A |
Question
Which row correctly describes the bonding type and melting point of carbon and carbon dioxide?
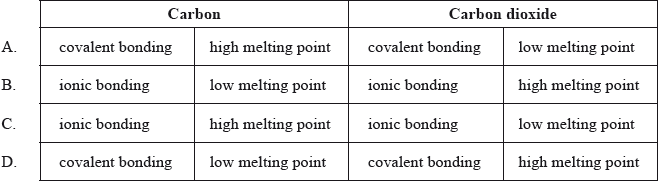
Markscheme
A
Examiners report
One G2 comment stated that none of the answers were correct for this question and stated that the question was not clear as there was no mention of intermolecular force considerations. The question itself simply involved looking at two features for both substances, carbon and carbon dioxide – firstly whether the bonding is ionic or covalent and secondly whether the melting point is high or low. It was not necessary to include intermolecular force considerations to answer this question, as clearly from the choices given A is the most appropriate answer. Clearly both carbon and carbon dioxide involve covalent bonding and carbon will involve a high melting point (particularly in the case of the allotropes, graphite and diamond, though of course the melting points of graphite and diamond are higher than that of fullerene) whereas the melting point for carbon dioxide will be low. 69% of candidates gave A as the correct answer.

