| Date | November 2014 | Marks available | 3 | Reference code | 14N.2.sl.TZ0.3 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Explain | Question number | 3 | Adapted from | N/A |
Question
Graphite has a layered structure of carbon atoms. A section of the structure is shown below.
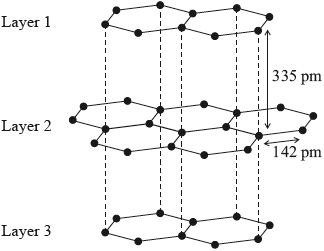
Explain why the distance between adjacent carbon atoms within a layer is shorter than the distance between layers.
Graphite is used as a lubricant. Discuss two other uses of graphite with reference to its layered structure.
Markscheme
(attraction within layer/between carbons) covalent bonding / sharing of electrons;
(attraction between layers) van der Waals’/vdW/London/dispersion/LDF (forces) / temporary/instantaneous/induced dipoles;
bond/attraction within the layer stronger than bond/attraction between layers;
Two of the following pairs:
used as pencil (lead);
layers can flake off/slide off/break off/stick to paper / OWTTE;
M2 must contain concept of separation of layers, so do not award mark for phrases like “layers can slide over each other” on their own.
OR
used as carbon fibre / OWTTE;
bonding within layer is strong / layers are extensive / layers are strong;
OR
used as electrodes/conductor/in batteries;
has mobile/free/delocalized electrons (between layers) / electricity flows parallel to layers;
OR
used for thermal insulation;
vibrations are not easily passed between layers;
Accept other valid uses of graphite along with a suitable explanation.
Examiners report
The better candidates had little difficulty in correctly identifying the forces both within and between the layers of graphite and pointing out that stronger forces produce shorter bonds. In Part (b) most candidates knew of the use of graphite in pencils, though the property identified was often the ability of the layers to slide over each other rather than their ability to break free, and many students struggled for a second use, though a significant number mentioned and correctly explain its electrical conductivity.
The better candidates had little difficulty in correctly identifying the forces both within and between the layers of graphite and pointing out that stronger forces produce shorter bonds. In Part (b) most candidates knew of the use of graphite in pencils, though the property identified was often the ability of the layers to slide over each other rather than their ability to break free, and many students struggled for a second use, though a significant number mentioned and correctly explain its electrical conductivity.

