| Date | May 2015 | Marks available | 4 | Reference code | 15M.2.sl.TZ1.6 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Deduce and Explain | Question number | 6 | Adapted from | N/A |
Question
Across period 3, elements increase in atomic number, decrease in atomic radius and increase in electronegativity.
Define the term electronegativity.
Explain why the atomic radius of elements decreases across the period.
State the equations for the reactions of sodium oxide with water and phosphorus(V) oxide with water.
Suggest the pH of the solutions formed in part (c) (i).
Describe three tests that can be carried out in the laboratory, and the expected results, to distinguish between \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ HCl(aq)}}\) and \({\text{0.10 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ C}}{{\text{H}}_{\text{3}}}{\text{COOH(aq)}}\).
Explain whether BF3 can act as a Brønsted-Lowry acid, a Lewis acid or both.
Describe the bonding and structure of sodium chloride.
State the formula of the compounds formed between the elements below.
Sodium and sulfur:
Magnesium and phosphorus:
Covalent bonds form when phosphorus reacts with chlorine to form \({\text{PC}}{{\text{l}}_{\text{3}}}\). Deduce the Lewis (electron dot) structure, the shape and bond angle in \({\text{PC}}{{\text{l}}_{\text{3}}}\) and explain why the molecule is polar.
Lewis (electron dot) structure:
Name of shape:
Bond angle:
Explanation of polarity of molecule:
Markscheme
ability of atom/nucleus to attract bonding/shared pair of electrons / attraction of nucleus for bonding/shared pair of electrons;
Do not accept “element” instead of “atom/nucleus”.
Do not accept “electrons” alone.
increasing nuclear charge/increasing number of protons / increased attraction of (valence) electrons to nucleus;
electrons added are in same (outer) energy level;
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O(s)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{2NaOH(aq)}}\);
Accept \(N{a_2}O(s) + {H_2}O(l) \to 2N{a^ + }(aq) + 2O{H^ - }(aq)\).
\({{\text{P}}_4}{{\text{O}}_{10}}{\text{(s)}} + {\text{6}}{{\text{H}}_2}{\text{O(l)}} \to {\text{4}}{{\text{H}}_3}{\text{P}}{{\text{O}}_3}{\text{(aq)}}\);
Accept \({P_2}{O_5}(s) + 3{H_2}O(l) \to 2{H_3}P{O_4}(aq)\).
Accept \({P_4}{O_{10}}(s) + 6{H_2}O(l) \to 4{H^ + }(aq) + 4{H_2}PO_4^ - (aq)\).
Ignore state symbols.
NaOH: > 7;
Accept any pH greater than 7.
H3PO4: < 7;
Accept any pH less than 7.
Award [1 max] if stated that “NaOH alkali/basic and H3PO4 acidic”, but pH values not given.
measuring electrical conductivity and strong acids have greater electrical
conductivity/weak acids have lower electrical conductivity;
Do not accept conductivity for electrical conductivity.
Accept explanation in terms of lightbulb in circuit.
measure pH/use universal indicator and pH higher for weak acid/pH lower for strong acid;
conduct titration with a strong base and equivalence point higher for weak acid / buffer region for weak acid;
adding a reactive metal/carbonate/hydrogen carbonate and stronger effervescence/faster reaction with strong acids;
Accept converse argument.
Accept correct example.
adding a strong base and strong acid would increase more in temperature/weak acids increase less in temperature;
Accept correct example.
Award [1 max] for three suitable tests without correct results.
Accept specific examples with given strong acid and weak acid.
Accept “addition of AgNO3 (aq) and white precipitate with HCl (aq)”.
Do not accept “smell”.
Lewis acid (only);
electron pair acceptor / not a proton donor;
Bonding: (electrostatic) attraction between oppositely charged ions;
Do not accept ionic bonding without some description.
Structure: lattice/giant structure of ions / each \({\text{N}}{{\text{a}}^ + }\) surrounded by \({\text{6 C}}{{\text{l}}^ - }\) (and vice-versa);
\({\text{N}}{{\text{a}}_2}{\text{S}}\);
\({\text{M}}{{\text{g}}_3}{{\text{P}}_2}\);
Lewis structure:
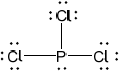 ;
;
Accept any combination of lines, dots or crosses to represent electron pairs.
Do not award the mark if lone pairs are missing.
Name of shape:
(trigonal/triangular) pyramidal;
Bond angle:
\( < 109.5^\circ \);
Accept any value within the range 100°−109°.
Literature value is 100°.
Explanation of polarity:
dipoles do not cancel (as molecule is not symmetrical) / there is a net dipole (as molecule is not symmetrical) / unsymmetrical distribution of charge;
Accept suitable labelled diagram.
No ECF if original structure is incorrect.
Examiners report
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.
This was by far the most popular question. As before the definition was poorly done and many students defined electronegativity as just attraction for electrons or energy change in gaining an electron. However, many could at least half explain why the atomic radius decreased. In (c) some students could write a correct equation for the addition of sodium oxide to water but very few could correctly write an equation for phosphorous(V) oxide with water, following on few could then correctly state a sensible pH for the solutions formed. Suggesting methods to distinguish between strong and weak acids was reasonably well answered but many student lost marks for the imprecision in their answers. Stating "see if it conducts" and "add pH paper" were common answers without predictions of the expected results. Identification of \({\text{B}}{{\text{F}}_{\text{3}}}\) as a Lewis acid was not always explained well as students mixed up proton donation and electron pair donation. In (f) the description of the bonding and structure of sodium chloride was not well done, although there were a few strong candidates who had little problems with this question. Most candidates could correctly state the ionic formulae though. The last part of this question asked for a Lewis structure of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and most did this well, although some forgot the lone pairs on the chlorine atoms. Most could then correctly state a bond angle although there were a number of candidates who stated 120°. Few candidates could explain why the molecule was polar.

