| Date | May 2011 | Marks available | 1 | Reference code | 11M.1.sl.TZ2.13 |
| Level | SL | Paper | 1 | Time zone | TZ2 |
| Command term | Identify | Question number | 13 | Adapted from | N/A |
Question
Lewis structures are represented in different ways in different parts of the world. Two ways of drawing the Lewis structure for \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) are shown below.
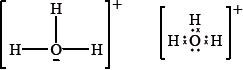
Which statement is correct about \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\)?
A. The ion has a tetrahedral shape.
B. The H–O–H bond angle is 120°.
C. The H–O–H bond angle is 90°.
D. The ion has a trigonal pyramidal shape.
Markscheme
D
Examiners report
One respondent stated that as the hydronium cation involves dative covalent bonding it would have been better if the dot-cross representation would have reflected this, which is a valid point. However, this did not stop candidates answering the question and 72.31% of candidates got the correct answer, namely that the ion has a trigonal pyramidal shape i.e. D.
Syllabus sections
- 17N.2.sl.TZ0.3b: Predict with a reason, whether the molecule PF3 is polar or non-polar.
- 17N.2.sl.TZ0.3a: Draw the Lewis (electron dot) structures of PF3 and PF4+ and use the VSEPR theory to deduce...
- 17N.2.hl.TZ0.4b: Predict whether the molecules PF3 and PF5 are polar or non-polar.
- 17N.2.hl.TZ0.4a: Draw the Lewis (electron dot) structures of PF3 and PF5 and use the VSEPR theory to deduce...
- 17M.2.hl.TZ1.5c: Ammonia reacts reversibly with...
- 17M.2.hl.TZ1.5a: Estimate the H−N−H bond angle in methanamine using VSEPR theory.
- 17M.2.hl.TZ2.4b.ii: Deduce one resonance structure of ozone and the corresponding formal charges on each oxygen...
- 17M.2.sl.TZ2.4b: Deduce the Lewis (electron dot) structures of ozone.
- 17M.2.sl.TZ2.3b: Deduce the Lewis (electron dot) structure and molecular geometry of PCl3.
- 17M.1.sl.TZ2.11: What are the approximate bond angles and structure of crystalline SiO2?
- 17M.2.hl.TZ1.5b: State the electron domain geometry around the nitrogen atom and its hybridization...
- 17M.2.sl.TZ1.4a: Estimate the H−N−H bond angle in methanamine using VSEPR theory.
- 17M.1.hl.TZ1.12: Which combination describes the bonding and structure in benzoic acid, C6H5COOH?
- 17M.1.sl.TZ1.12: Which correctly states the strongest intermolecular forces in the compounds below?
- 17M.1.sl.TZ1.11: Which combination describes the sulfate(IV) ion, SO32– (also known as sulfite ion)?
- 17M.1.sl.TZ1.9: A substance has the following properties: What is the most probable structure of this...
- 16N.3.sl.TZ0.6c: (i) Suggest why incomplete combustion of plastic, such as polyvinyl chloride, is common in...
- 16N.2.hl.TZ0.2e: Outline why all the C–O bond lengths in the ethanedioate ion are the same length and suggest...
- 16N.2.hl.TZ0.2d: Draw the Lewis (electron dot) structure of the ethanedioate ion, –OOCCOO–.
- 16N.2.sl.TZ0.2d: The Lewis (electron dot) structure of the ethanedioate ion is shown below. Outline why all...
- 16N.1.sl.TZ0.12: Which substance has a giant covalent structure?
- 16N.1.sl.TZ0.9: Which pair of molecules has the same bond angles? A. PCl3 and BCl3 B. SO2 and CO2 C....
- 16M.2.hl.TZ0.3c: One of the intermediates in the reaction between nitrogen monoxide and hydrogen is dinitrogen...
- 16M.2.hl.TZ0.1a: (i) Draw a Lewis (electron dot) structure of phosphine. (ii) State the hybridization of the...
- 16M.2.sl.TZ0.1b: Phosphine is usually prepared by heating white phosphorus, one of the allotropes of...
- 16M.2.sl.TZ0.1a: (i) Draw a Lewis (electron dot) structure of phosphine. (ii) Outline whether you expect the...
- 16M.1.sl.TZ0.11: Which compound has resonance structures? A. C6H12 B. CH3CHO C. NaBr D. Na2CO3
- 15M.1.hl.TZ1.11: Which substance has the following properties? • Low melting point • Very soluble in...
- 15M.1.hl.TZ2.10: Which diagrams can be used to represent the Lewis (electron dot) structure of boron...
- 15M.2.hl.TZ1.1d: Deduce the Lewis (electron dot) structure of ethanedioic acid, \({\text{HOOC–COOH}}\).
- 15M.2.hl.TZ1.2b.ii: Predict whether phosphorus(V) oxide and sodium oxide conduct electricity in their solid and...
- 15M.2.hl.TZ1.2b.i: Explain why the melting point of phosphorus(V) oxide is lower than that of sodium oxide in...
- 15M.2.hl.TZ1.5g: Identify three allotropes of carbon and describe their structures.
- 15M.1.sl.TZ1.10: Which molecules react to form a dative covalent (coordinate) bond? A. ...
- 15M.1.sl.TZ1.11: What describes the relationship between diamond, graphite and \({{\text{C}}_{{\text{60}}}}\)...
- 15M.1.sl.TZ1.9: What describes the structure of silicon and silicon dioxide?
- 15M.1.sl.TZ2.10: Which species contain a dative covalent (coordination or coordinate) bond? I. Carbon...
- 15M.1.sl.TZ2.11: Which combination of shape and bond angle best describes a molecule of sulfur dioxide,...
- 15M.2.sl.TZ1.1e: Deduce the Lewis (electron dot) structure of ethanedioic acid, HOOC−COOH.
- 15M.2.sl.TZ1.6g: Covalent bonds form when phosphorus reacts with chlorine to form...
- 15M.2.sl.TZ2.6a.i: Draw the Lewis (electron dot) structure of chloromethane.
- 15M.2.sl.TZ2.6a.ii: Predict the shape of the chloromethane molecule and the H–C–H bond...
- 15M.2.sl.TZ2.6a.iii: Explain why chloromethane is a polar molecule.
- 14M.1.hl.TZ1.11: A solid has a melting point of 1582 °C and does not dissolve in water. It does not conduct...
- 14M.2.hl.TZ1.1d: Magnesium sulfate is one of the products formed when acid rain reacts with dolomitic...
- 14M.2.hl.TZ1.5a: (i) State the changes in the acid-base nature of the oxides across period 3 (from...
- 14M.2.hl.TZ2.4b: State the shape of the ozone molecule and estimate the bond angle. Shape: Bond angle:
- 14M.1.sl.TZ1.11: What is the shape and the bond angle of the molecule \({\text{B}}{{\text{F}}_{\text{3}}}\)?
- 14M.1.sl.TZ2.12: Which pair has the same bond angles? A. ...
- 14M.2.sl.TZ1.1e: (i) State the equation for the reaction of sulfuric acid with magnesium...
- 14M.1.sl.TZ2.13: Which diagram represents the bonding in \({\text{Si}}{{\text{O}}_{\text{2}}}\)?
- 14M.2.sl.TZ2.4c.v: Draw the Lewis (electron dot) structure of chloric(I) acid.
- 14M.2.sl.TZ2.4c.vi: Predict the H–O–Cl bond angle in this molecule and explain this in terms of the valence shell...
- 14M.3.sl.TZ1.24d: State the formula and deduce the shape of the positive ion (cation) formed when...
- 14N.1.hl.TZ0.13: Which group of ions and molecules has delocalized electrons in all the species? A. ...
- 14N.2.hl.TZ0.5a: Identify the type of attraction represented by the dotted lines shown between the layers.
- 14N.2.hl.TZ0.5b: Graphite is used as a lubricant. Discuss two other uses of graphite with reference to its...
- 14N.1.sl.TZ0.13: Which species contains a bond angle of approximately 107°? A. ...
- 14N.1.sl.TZ0.9: Which species contains a dative covalent (coordinate) bond? A. HCN B. ...
- 14N.2.sl.TZ0.3a: Explain why the distance between adjacent carbon atoms within a layer is shorter than the...
- 14N.2.sl.TZ0.3b: Graphite is used as a lubricant. Discuss two other uses of graphite with reference to its...
- 14N.2.sl.TZ0.6e: (i) Deduce the Lewis structure of \({\text{PH}}_4^ + \). (ii) Predict, giving a...
- 13N.2.hl.TZ0.4b: State the name given to species that bond to a central metal ion, and identify the type of...
- 13N.2.hl.TZ0.5h: Deduce the N–N–N bond angle in trinitramide and explain your reasoning.
- 13N.2.hl.TZ0.5i: Predict, with an explanation, the polarity of the trinitramide molecule.
- 13N.2.hl.TZ0.6e.i: Compare the properties of the three oxides by completing the table below.
- 13N.2.hl.TZ0.6e.iii: As well as the oxide above, sodium forms a peroxide that contains the peroxide ion,...
- 13N.1.sl.TZ0.12: The Lewis (electron dot) structure of aspirin is represented below. What are the...
- 13N.2.sl.TZ0.4e: Deduce the N–N–N bond angle in trinitramide and explain your reasoning.
- 13N.2.sl.TZ0.4f: Predict, with an explanation, the polarity of the trinitramide molecule.
- 13M.2.hl.TZ1.6c.v: Compare the melting points of \({\text{PC}}{{\text{l}}_{\text{3}}}\) and...
- 13M.1.sl.TZ1.13: Which combination best describes the type of bonding present and the melting point of silicon...
- 13M.2.sl.TZ1.6c.i: Deduce the Lewis structures of \({\text{N}}{{\text{H}}_{\text{3}}}\) and...
- 13M.2.sl.TZ1.6c.iii: Compare the shapes of the two molecules and explain the difference using valence shell...
- 13M.2.sl.TZ1.6c.iv: Predict and explain whether the molecules \({\text{N}}{{\text{H}}_{\text{3}}}\) and...
- 13M.2.hl.TZ2.3b: State the type of bonding between platinum and nitrogen in carboplatin.
- 13M.2.hl.TZ2.5b.ii: State and explain the Cl–P–Cl bond angle in PCl3.
- 13M.1.sl.TZ2.11: Which statements about graphite are correct? I. Carbon atoms are held in layers with...
- 13M.1.sl.TZ2.13: Which statements about the structure and bonding of silicon dioxide are correct?
- 13M.2.sl.TZ2.5b.i: Deduce the Lewis (electron dot) structure and predict the shape of each molecule, using the...
- 13M.2.sl.TZ2.5b.ii: State and explain the F–S–F bond angle in SF2.
- 13M.2.sl.TZ2.5b.iii: Deduce whether each of the three molecules is polar or non-polar, giving your reason in each...
- 12N.1.sl.TZ0.12: Diamond, C60 fullerene and graphite are allotropes of carbon. Which statements are correct...
- 12N.1.sl.TZ0.13: Which statement about the physical properties of substances is correct? A. The only...
- 12N.2.sl.TZ0.2b: (i) Determine \(\Delta H\), the enthalpy change of the reaction, in...
- 12N.2.sl.TZ0.5b.ii: The Lewis (electron dot) structure of nitrous acid is given below. Identify which...
- 12N.2.sl.TZ0.5b.v: Ammonia, NH3, is a weak base. Deduce the Lewis (electron dot) structure of NH3. State the...
- 12N.2.sl.TZ0.5b.iii: Deduce the approximate value of the hydrogen-oxygen-nitrogen bond angle in nitrous acid and...
- 10N.1.hl.TZ0.12: Which molecule has an octahedral shape? A. SF6 B. PCl5 C. XeF4 D. BF3
- 10N.2.hl.TZ0.7d: The reaction between \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}{\text{(aq)}}\) and...
- 10N.1.sl.TZ0.11: Which species contain a dative covalent bond? I. HCHO II. CO III. ...
- 10N.2.sl.TZ0.4f: (i) Identify the type of reaction that occurs. (ii) Predict the value of the H–N–H...
- 09N.1.hl.TZ0.11: What is the bond angle in the \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) ion? A. ...
- 09N.1.hl.TZ0.13: How many atoms is each carbon directly bonded to in its allotropes?
- 09N.1.sl.TZ0.11: How many non-bonding pairs of electrons are there in a nitrogen molecule? A. 0 B. ...
- 09N.1.sl.TZ0.12: Which molecule contains a bond angle of approximately 120°? A. ...
- 09N.2.sl.TZ0.2: PF3, SF2 and SiF4 have different shapes. Draw their Lewis structures and use the VSEPR theory...
- 10M.2.sl.TZ1.3a.i: Draw the Lewis structure for chloroethene and predict the H–C–Cl bond angle.
- 10M.1.sl.TZ2.14: Which compound has a covalent macromolecular (giant covalent) structure? A. MgO(s) B. ...
- 10M.1.sl.TZ2.11: What is the shape of the ammonia molecule, \({\text{N}}{{\text{H}}_{\text{3}}}\)? A. ...
- 10M.1.sl.TZ2.12: Which molecule is polar? A. ...
- 10M.2.sl.TZ2.7a.ii: Deduce the Lewis structure of chloroethene and identify the formula of the repeating unit of...
- 09M.1.hl.TZ1.11: Which molecule contains a dative covalent (coordinate) bond? A. HCN B. ...
- 09M.2.hl.TZ1.6c.iii: Deduce all the bond angles present in propene.
- 09M.1.sl.TZ1.14: Which is the best description of the bonding present in silicon dioxide,...
- 09M.2.sl.TZ1.6b.i: \({\text{C}}{{\text{O}}_{\text{2}}}\)
- 09M.2.sl.TZ1.6b.ii: \({\text{CO}}_3^{2 - }\)
- 09M.2.sl.TZ1.6b.iii: \({\text{BF}}_4^ - \)
- 09M.2.hl.TZ2.5b.ii: \({\text{NO}}_2^ + \)
- 09M.2.sl.TZ2.7a.iii: Describe the structure and bonding in silicon dioxide and carbon dioxide.
- 09M.2.sl.TZ2.7b.i: Draw the Lewis structure of NH3, state its shape and deduce and explain the H–N–H bond angle...
- 11M.1.hl.TZ1.14: The Lewis structure of \({\text{S}}{{\text{O}}_{\text{2}}}\) is given below. What is the...
- 11M.2.hl.TZ1.6f.i: Explain the electrical conductivity of molten sodium oxide and liquid sulfur trioxide.
- 11M.1.sl.TZ1.11: How do the bond angles in \({\text{C}}{{\text{H}}_{\text{4}}}\),...
- 11M.1.sl.TZ1.9: What is the correct Lewis structure for hypochlorous acid, a compound containing chlorine,...
- 11M.2.sl.TZ1.5b.ii: sodium oxide has a higher melting point than sulfur trioxide.
- 11M.2.sl.TZ1.7d.i: Draw the Lewis structure of \({\text{C}}{{\text{O}}_{\text{2}}}\) and predict its shape and...
- 11M.2.sl.TZ1.7d.iii: Explain why silicon dioxide is a solid and carbon dioxide is a gas at room temperature.
- 11M.2.sl.TZ1.7d.ii: Describe the structure and bonding in \({\text{Si}}{{\text{O}}_{\text{2}}}\).
- 11M.2.sl.TZ1.7c: Describe and compare three features of the structure and bonding in the three allotropes of...
- 11M.1.sl.TZ2.10: Which molecule has a non-bonding (lone) pair of electrons on the central atom? A. ...
- 12M.1.hl.TZ2.9: Which species contain dative covalent bonds? I. CO II. ...
- 12M.1.sl.TZ2.12: The Lewis (electron dot) structure of paracetamol (acetaminophen) is: What are the...
- 12M.1.sl.TZ2.13: \({{\text{C}}_{{\text{60}}}}\) fullerene consists of a simple molecular structure. Silicon...
- 12M.2.sl.TZ2.5c: Graphite is used as a lubricant and is an electrical conductor. Diamond is hard and does not...
- 12M.2.sl.TZ2.6c: (i) When ammonium chloride, \({\text{N}}{{\text{H}}_{\text{4}}}{\text{Cl(aq)}}\), is...
- 11N.2.hl.TZ0.6b.i: State and explain the difference in the electrical conductivity in the liquid state of the...
- 11N.2.hl.TZ0.8e.i: Identify the type of bond present between \({\text{B}}{{\text{F}}_{\text{3}}}\) and...
- 11N.2.sl.TZ0.5c.ii: Explain why \({\text{PB}}{{\text{r}}_{\text{3}}}\) is a polar molecule.
- 11N.1.sl.TZ0.10: Which row correctly describes the bonding type and melting point of carbon and carbon dioxide?
- 11N.1.sl.TZ0.12: Which is the correct Lewis structure for ethene?
- 11N.2.sl.TZ0.1c.ii: The metal oxides from the second reaction then react with silicon dioxide to form a silicate...
- 11N.2.sl.TZ0.5c.i: For each of the species \({\text{PB}}{{\text{r}}_{\text{3}}}\) and HCHO: • deduce the...

