| Date | May 2012 | Marks available | 1 | Reference code | 12M.1.sl.TZ2.12 |
| Level | SL | Paper | 1 | Time zone | TZ2 |
| Command term | Question number | 12 | Adapted from | N/A |
Question
The Lewis (electron dot) structure of paracetamol (acetaminophen) is:
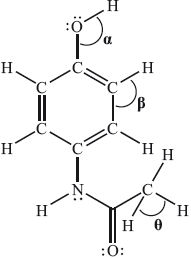
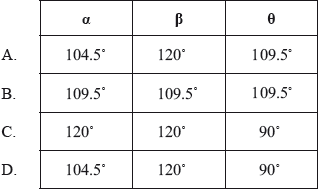
What are the approximate values of the bond angles?
Markscheme
A
Examiners report
In one G2 comment it was stated that since the question is about bond angles then it would be better to use a 3D representation of paracetamol. This was not the intention of the question. Candidates had to look at the number of negative charge centres (electron domains) around the two carbon atoms and the oxygen atom in order to relate this to the associated bond angle. In the case of the oxygen atom, there are four negative charge centres suggesting that the electron domain geometry is tetrahedral but the molecular geometry is actually v-shaped (bent). Due to the lone-pair/lone-pair repulsion, the actual bond angle is reduced from the ideal bond angle of 109.5° for \(\alpha \). For the two carbon atoms, one has three negative charge centres, implying a 120° bond angle and the other has four negative charge centres suggesting a 109.5° bond angle based on a tetrahedral molecular geometry around the carbon. 63.16% of candidates got the correct answer A. The question also had a reasonably good discrimination index of 0.55. Many candidates opted for D and simply took the bond angle based on the Lewis structure to be 90° for the H–C–H bond. This shows again the importance of introducing the 3D nature of molecules in the teaching of geometry as part of the teaching programme. Candidates should be exposed to constructing simple 3D molecules in class (and/or engaging with computer-aided visualizations if facilities allow) and candidates should understand the inherent differences between Lewis (electron dot) structures (which do not necessarily convey angular perspectives) and ball and stick type or other similar 3D representations. VSEPR theory should be employed as a useful model in bridging these two types of representations and this is especially important in looking at structures in the teaching of organic chemistry, where 2D structural formulas are often used.

