| Date | May 2009 | Marks available | 1 | Reference code | 09M.2.sl.TZ2.5 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Define | Question number | 5 | Adapted from | N/A |
Question
Consider the following equilibrium.
\[\begin{array}{*{20}{l}} {{\text{2S}}{{\text{O}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g)}} \rightleftharpoons {\text{2S}}{{\text{O}}_3}{\text{(g)}}}&{\Delta {H^\Theta } = - 198{\text{ kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}} \end{array}\]
Deduce the equilibrium constant expression, \({K_{\text{c}}}\), for the reaction.
State and explain the effect of increasing the temperature on the yield of sulfur trioxide.
State the effect of a catalyst on the value of \({K_{\text{c}}}\).
State and explain the effect of a catalyst on the position of equilibrium.
Define oxidation in terms of oxidation numbers.
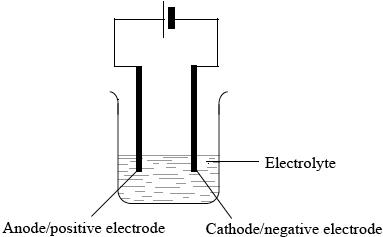
Describe using a labelled diagram, the essential components of an electrolytic cell.
Explain why solid sodium chloride does not conduct electricity but molten sodium chloride does.
Molten sodium chloride undergoes electrolysis in an electrolytic cell. For each electrode deduce the half-equation and state whether oxidation or reduction takes place. Deduce the equation of the overall cell reaction including state symbols.
Electrolysis has made it possible to obtain reactive metals such as aluminium from their ores, which has resulted in significant developments in engineering and technology. State one reason why aluminium is preferred to iron in many uses.
Outline two differences between an electrolytic cell and a voltaic cell.
Markscheme
\({\text{(}}{K_{\text{c}}} = {\text{)[S}}{{\text{O}}_{\text{3}}}{{\text{]}}^{\text{2}}}{\text{/[}}{{\text{O}}_{\text{2}}}{\text{][S}}{{\text{O}}_{\text{2}}}{{\text{]}}^{\text{2}}}\);
yield (of \({\text{S}}{{\text{O}}_{\text{3}}}\)) decreases;
forward reaction is exothermic / reverse/backwards reaction is endothermic / equilibrium shifts to absorb (some of) the heat;
Do not accept exothermic reaction or Le Châtelier’s Principle.
Do not allow ECF.
no effect;
no effect;
the rates of both the forward and reverse reactions increase equally;
increase in the oxidation number;
Annotated diagram of cell showing:
power supply/battery;
electrolyte;
cathode/negative electrode and anode/positive electrode;
(solid) ions in a lattice / ions cannot move;
(molten) ions mobile / ions free to move;
reduction occurs at the cathode/negative electrode and oxidation occurs at the anode/positive electrode;
Cathode/negative electrode: \({\text{N}}{{\text{a}}^ + } + {{\text{e}}^ - } \to {\text{Na}}\);
Anode/positive electrode: \({\text{2C}}{{\text{l}}^ - } \to {\text{C}}{{\text{l}}_2} + {\text{2}}{{\text{e}}^ - }/{\text{C}}{{\text{l}}^ - } \to \frac{1}{2}{\text{C}}{{\text{l}}_2} + {{\text{e}}^ - }\);
Award [1 max] if the two electrodes are not labelled/labelled incorrectly for the two half-equations.
Overall cell reaction: \({\text{N}}{{\text{a}}^ + }{\text{(1)}} + {\text{C}}{{\text{l}}^ - }{\text{(1)}} \to {\text{Na(1)}} + \frac{1}{2}{\text{C}}{{\text{l}}_2}{\text{(g)}}\)
Award [1] for correct equation and [1] for correct state symbols.
Allow NaCl(l) instead of Na+(l) and Cl–(l).
Al does not corrode/rust / Al is less dense/better conductor/more malleable;
Accept Al is a lighter (metal compared to Fe).
Accept converse argument.
electrolytic cell converts electrical energy to chemical energy and voltaic cell converts chemical energy to electrical energy / electrolytic cell uses electricity to carry out a (redox) chemical reaction and voltaic cell uses a (redox) chemical reaction to produce electricity / electrolytic cell requires a power supply and voltaic cell does not;
electrolytic cell involves a non-spontaneous (redox) reaction and voltaic cell involves a spontaneous (redox) reaction;
in an electrolytic cell, cathode is negative and anode is positive and vice-versa for a voltaic cell / electrolytic cell, anode is positive and voltaic cell, anode is negative / electrolytic cell, cathode is negative and voltaic cell, cathode is positive;
voltaic cell has two separate solutions and electrolytic cell has one solution / voltaic cell has salt bridge and electrolytic cell has no salt bridge;
electrolytic cell, oxidation occurs at the positive electrode/anode and voltaic cell, oxidation occurs at the negative electrode/anode and vice-versa;
Examiners report
Nearly all candidates deduced the equilibrium constant expression for the reaction given in (a) (i).
there were many good and complete answers here for (a) (ii). Some candidates did not state that the forward reaction was exothermic or the reverse reaction was endothermic, when trying to decide the effect of an increase in temperature on the yield of \({\text{S}}{{\text{O}}_{\text{3}}}\).
In (a) (iii) most candidates correctly stated that the catalyst would not have any effect on the value of \({K_{\text{c}}}\).
In part (iv) many candidates correctly stated that the catalyst would not have any effect on the position of equilibrium, but some did not explain why.
In (b) (i) some candidates defined oxidation as the loss of electrons but not in terms of oxidation numbers, as required by the question.
Some candidates described a voltaic cell instead of an electrolytic cell in (b) (ii). In some cases the electrodes were wrongly labelled or wrongly connected to the battery and the electrolyte was missing.
A large number of candidates stated that solid sodium chloride did not conduct electricity because it did not contain electrons in (iii). However some gave the correct answer indicating the free/moving ions as the particles responsible for the conductivity.
Part (b) (iv) was generally well answered. Most candidates lost a mark because they did not give the correct state symbols in the overall reaction.
Most candidates gave a correct answer as to why aluminium is preferred to iron in many uses in (b) (v).
There were very good answers indicating the main differences between an electrolytic cell and a voltaic cell in (vi).

