| Date | May 2014 | Marks available | 1 | Reference code | 14M.1.hl.TZ2.31 |
| Level | HL | Paper | 1 | Time zone | TZ2 |
| Command term | Question number | 31 | Adapted from | N/A |
Question
Which species are the oxidizing and reducing agents in the following reaction?
\[{\text{SO}}_3^{2 - }{\text{(aq)}} + {\text{Pb}}{{\text{O}}_2}{\text{(s)}} + {{\text{H}}_2}{\text{O(l)}} \to {\text{SO}}_4^{2 - }{\text{(aq)}} + {\text{Pb(OH}}{{\text{)}}_2}{\text{(s)}}\]
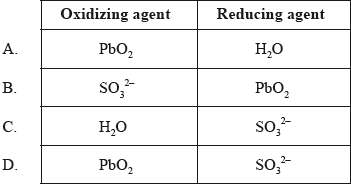
Markscheme
D
Examiners report
[N/A]
Syllabus sections
Show 165 related questions
- 17N.2.sl.TZ0.2e.i: Identify the strongest reducing agent in the given list.
- 17N.1.sl.TZ0.22: Which of the following is a redox reaction? A. 3Mg (s) + 2AlCl3 (aq) → 2Al (s) + 3MgCl2...
- 17N.1.sl.TZ0.21: What are the oxidation states of chromium in (NH4)2Cr2O7 (s) and Cr2O3 (s)?
- 17N.2.hl.TZ0.7d: Identify the best reducing agent in the table above.
- 17N.1.hl.TZ0.30: Consider the following half-equations: I2 (s) + 2e– \( \rightleftharpoons \) 2I– (aq) ...
- 17M.3.sl.TZ2.5b.ii: Deduce the redox equation for the reaction of nickel(II) chloride solution with the metal...
- 17M.3.sl.TZ2.5b.i: Nickel is also used as a catalyst. It is processed from an ore until nickel(II) chloride...
- 17M.2.sl.TZ2.4a.iv: Deduce, giving a reason, which species is the reducing agent.
- 17M.2.sl.TZ2.4a.iii: Determine the oxidation state of nitrogen in the two reactants.
- 17M.2.sl.TZ2.2a.ii: Deduce the overall redox equation for the reaction between acidic Sn2+(aq) and Cr2O72–(aq),...
- 17M.2.sl.TZ2.2a.i: State the oxidation half-equation.
- 17M.1.hl.TZ2.36: Which compounds can be reduced? I. C2H4II. CH3COOHIII. CH3CHO A. I and II...
- 17M.1.hl.TZ2.28: Which element is reduced in the following decomposition? (NH4)2Cr2O7(s) → N2(g) + Cr2O3(s) +...
- 17M.1.sl.TZ2.22: Which of the following is not a redox reaction? A. CH4(g) + Cl2(g) → CH3Cl(g) +...
- 17M.1.sl.TZ2.21: Which element is reduced in the following decomposition? (NH4)2Cr2O7(s) → N2(g) + Cr2O3(s) +...
- 17M.1.sl.TZ2.19: Which of the following does not react with dilute HCl(aq)? A. Na2CO3 B. Cu C. ...
- 17M.2.hl.TZ1.4d: Suggest why experiments involving tetracarbonylnickel are very hazardous.
- 17M.2.hl.TZ1.4b: The nickel obtained from another ore, nickeliferous limonite, is contaminated with iron. Both...
- 17M.2.hl.TZ1.4a: Formulate an equation for the oxidation of nickel(II) sulfide to nickel(II) oxide.
- 17M.2.hl.TZ1.3c.i: Formulate an equation for the reaction between VO2+(aq) and V2+(aq) in acidic solution to...
- 17M.2.hl.TZ1.3b.ii: Identify, from the table, a non-vanadium species that could convert...
- 17M.2.hl.TZ1.3b.i: Identify, from the table, a non-vanadium species that can reduce VO2+(aq) to V3+(aq) but no...
- 17M.2.sl.TZ1.3b: Formulate an equation for the reaction between VO2+(aq) and V2+(aq) in acidic solution to...
- 17M.2.sl.TZ1.3a: Determine the oxidation state of vanadium in each of the following species.
- 17M.2.sl.TZ1.1a.i: Suggest how the change of iodine concentration could be followed.
- 17M.1.hl.TZ1.29: A reaction takes place when a rechargeable battery is used: Pb(s) + PbO2(s) + 4H+(aq) +...
- 17M.1.hl.TZ1.28: Which change represents oxidation? A. HClO4 to HClO3 B. N2 to NH3 C. N2O to...
- 17M.1.sl.TZ1.22: What is the oxidation half-equation in the redox reaction? 2S2O32–(aq) + I2(aq) → S4O62–(aq)...
- 17M.1.sl.TZ1.21: What is the order of decreasing reactivity of the metals (most reactive first)? Zn(s) +...
- 16N.3.sl.TZ0.1c: Despite widespread improvements in the provision of safe drinking water, the sale of bottled...
- 16N.2.hl.TZ0.4i: Magnesium chloride can be electrolysed. (i) Deduce the half-equations for the reactions at...
- 16N.2.sl.TZ0.4g: Magnesium chloride can be electrolysed. Deduce the half-equations for the reactions at each...
- 16N.2.sl.TZ0.1b: Determine the average oxidation state of carbon in ethene and in...
- 16N.1.sl.TZ0.21: Which is a correct statement for the reaction below? 2MnO4-(aq) + 6H+(aq) + 5NO2-(aq) →...
- 16N.1.sl.TZ0.17: Which experimental methods could be used to observe the progress of the following...
- 16M.2.hl.TZ0.1b: Phosphine is usually prepared by heating white phosphorus, one of the allotropes of...
- 16M.2.sl.TZ0.1b: Phosphine is usually prepared by heating white phosphorus, one of the allotropes of...
- 16M.1.hl.TZ0.30: Applying IUPAC...
- 16M.1.sl.TZ0.21: Applying IUPAC rules, what is the name of MnO2? A. Magnesium(II) oxide B. Manganese(II)...
- 15M.1.hl.TZ1.30: Which represents a redox reaction? A. ...
- 15M.1.hl.TZ2.30: Which is a redox reaction? A. ...
- 15M.2.hl.TZ1.6e: Bromine reacts with aqueous sodium...
- 15M.2.hl.TZ1.7a.ii: Determine the oxidation number of carbon in ethanol and ethanal. Ethanol: Ethanal:
- 15M.2.hl.TZ1.7a.iii: Deduce the half-equation for the oxidation of ethanol to ethanal.
- 15M.2.hl.TZ1.7a.iv: Deduce the overall redox equation for the reaction of ethanol to ethanal with acidified...
- 15M.2.hl.TZ2.8b.i: State the name of \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\).
- 15M.2.hl.TZ2.8c.i: Chromium forms the complex ion...
- 15M.2.hl.TZ2.8d.i: The dichromate ion,...
- 15M.2.hl.TZ2.8d.ii: Explain in terms of oxidation numbers whether iodine is oxidized or reduced in part (d) (i).
- 15M.1.sl.TZ1.23: Which represents a redox reaction? A. ...
- 15M.1.sl.TZ1.24: Which species can oxidize ethanol to ethanoic acid? A. \({{\text{I}}^ - }\) B. ...
- 15M.1.sl.TZ2.23: What are the oxidation states of each element in...
- 15M.1.sl.TZ2.24: What is the coefficient for I– when the following equation is balanced using the smallest...
- 15M.2.sl.TZ1.2d: Bromine reacts with aqueous sodium...
- 15M.2.sl.TZ1.7c.iii: Deduce the half-equation for the oxidation of ethanol to ethanal.
- 15M.2.sl.TZ1.7c.ii: Determine the oxidation number of carbon in ethanol and ethanal. Ethanol: Ethanal:
- 15M.2.sl.TZ1.7c.iv: Deduce the overall redox equation for the reaction of ethanol to ethanal with acidified...
- 14M.2.hl.TZ1.6a: Alcohols with the molecular formula...
- 14M.2.hl.TZ2.5c: (i) Deduce a balanced equation for the reaction between the chlorate(I) ion and sulfur...
- 14M.1.sl.TZ2.23: What are the correct oxidation numbers of chromium in...
- 14M.2.sl.TZ1.6a: Define oxidation in terms of electron transfer.
- 14M.1.sl.TZ1.23: At which side of the equation are electrons, \({{\text{H}}^ + }\) ions and...
- 14M.1.sl.TZ1.24: What are the correct names for \({\text{KMn}}{{\text{O}}_{\text{4}}}\) and...
- 14M.2.sl.TZ1.6b: (i) Deduce the oxidation number of chromium in...
- 14M.2.sl.TZ2.4d: (i) Deduce the coefficients required to balance the half-equations given below. ___...
- 14M.3.sl.TZ1.16b: (i) \(1.50 \times {10^{ - 4}}{\text{ mol}}\) of \({{\text{I}}^ - }{\text{(aq)}}\) was...
- 14N.2.hl.TZ0.10a: (i) Identify whether half-equation 1 represents oxidation or reduction, giving a reason...
- 14N.3.hl.TZ0.24a: In an acidic soil, nitrate ions may undergo reduction to form ammonium ions. Deduce a...
- 14N.1.sl.TZ0.23: Which species of vanadium has a different oxidation number from the rest? A. ...
- 14N.1.sl.TZ0.24: Which statement is correct for the following...
- 14N.2.sl.TZ0.8a: (i) Identify whether half-equation 1 represents oxidation or reduction, giving a reason...
- 13N.1.hl.TZ0.31: What is the name of \({\text{Mn}}{{\text{O}}_{\text{2}}}\)? A. Manganese(II) oxide B. ...
- 13N.1.hl.TZ0.32: Consider the following...
- 13N.2.hl.TZ0.6b.i: State the initial and final oxidation numbers of titanium and hence deduce whether it is...
- 13N.3.hl.TZ0.9b.iv: The reducing agent in the cytochrome oxidase reaction is a species that can be denoted as...
- 13N.1.sl.TZ0.24: Consider the following...
- 13N.2.sl.TZ0.5b.iii: Deduce which of the six species is the strongest oxidizing agent.
- 13N.1.sl.TZ0.23: What is the name of...
- 13N.2.sl.TZ0.5a.i: State the initial and final oxidation numbers of titanium and hence deduce whether it is...
- 13N.2.sl.TZ0.5b.i: Deduce which of the species would react with titanium metal.
- 13N.2.sl.TZ0.5b.ii: Deduce the balanced equation for this reaction.
- 13M.2.hl.TZ1.4e.i: The standard electrode potential for the half-equation involving ethanedioic acid...
- 13M.2.hl.TZ1.4d: Deduce the half-equation involving ethanedioic acid.
- 13M.2.hl.TZ1.7b.iv: Deduce the oxidation number of iron in...
- 13M.1.sl.TZ1.23: Which statement is correct about a reducing agent? A. It is reduced by gaining...
- 13M.1.sl.TZ1.24: What is the correct increasing order of reactivity of the metals X, Y and Z based on the...
- 13M.2.sl.TZ1.4b: Calculate the change in oxidation numbers of carbon and...
- 13M.2.sl.TZ1.4a: Define oxidation in terms of electron transfer.
- 13M.2.sl.TZ1.4c: Identify the oxidizing and reducing agents. Oxidizing agent: Reducing agent:
- 13M.3.sl.TZ1.D2b.i: State the chemical formula for potassium dichromate(VI).
- 13M.3.sl.TZ1.D2b.iii: State the oxidation number of chromium in the product.
- 13M.2.hl.TZ2.7b.ii: Deduce the oxidizing and reducing agents in this reaction. Oxidizing agent: Reducing...
- 13M.2.hl.TZ2.7a: Define oxidation in terms of oxidation number.
- 13M.2.hl.TZ2.7b.i: Deduce the balanced chemical equation for the redox reaction of copper, Cu(s), with nitrate...
- 13M.2.hl.TZ2.7c.iii: Deduce a balanced chemical equation, including state symbols, for the overall reaction which...
- 13M.1.sl.TZ2.23: Which statement describes a reducing agent? A. It is reduced and gains electrons. B. ...
- 13M.1.sl.TZ2.24: Which is the oxidizing agent in the following...
- 13M.2.sl.TZ2.1d.i: Define the term reduction in terms of electrons.
- 13M.2.sl.TZ2.1d.ii: Deduce the oxidation number of manganese in the \({\text{MnO}}_{\text{4}}^ - {\text{(aq)}}\)...
- 13M.2.sl.TZ2.1f.i: One titration was abandoned because a brown precipitate, manganese(IV) oxide, formed. State...
- 12N.1.sl.TZ0.23: What is the correct systematic name of MnO2? A. Manganese(II) oxide B. ...
- 12N.2.sl.TZ0.5a: (i) Define oxidation and reduction in terms of electron loss or...
- 10N.1.hl.TZ0.30: Consider the following...
- 10N.1.hl.TZ0.31: The following equations indicate reactions that occur...
- 10N.1.sl.TZ0.23: Consider the following...
- 10N.2.sl.TZ0.6b: (i) Define oxidation in terms of oxidation numbers. (ii) Deduce the oxidation states...
- 10N.2.sl.TZ0.6g: Nitric acid reacts with silver in a redox reaction. __ \({\text{Ag(s)}} + \) __...
- 09N.1.hl.TZ0.29: Which compound contains nitrogen with an oxidation number of +3? A. NH4Cl B. ...
- 09N.1.sl.TZ0.24: Which are redox reactions? I. ...
- 09N.2.sl.TZ0.1a: Deduce the balanced redox equation for this reaction in acidic solution.
- 09N.2.sl.TZ0.1c: Calculate the amount, in moles, of \({\text{MnO}}_4^ - \) used in the titration.
- 09N.2.sl.TZ0.4b: Both \({{\text{C}}_{\text{5}}}{{\text{H}}_{{\text{12}}}}\) and...
- 09N.2.sl.TZ0.1b: Identify the reducing agent in the reaction.
- 09N.3.sl.TZ0.C4a: State the change in oxidation number of the cadmium and deduce if it is acting as the...
- 09N.3.sl.TZ0.E2a: State what is meant by the term biochemical oxygen demand (BOD).
- 10M.2.sl.TZ1.1: (a) (i) Deduce the change in the oxidation numbers of copper and nitrogen in step...
- 10M.2.sl.TZ1.5b: (i) Deduce the order of reactivity of the four metals, cadmium, nickel, silver and zinc...
- 10M.1.sl.TZ2.24: In which species does sulfur have an oxidation number of 0? A. ...
- 10M.1.sl.TZ2.25: What is the reducing agent in the reaction...
- 10M.2.sl.TZ2.5c: (i) Determine the oxidation number of lead in Pb,...
- 09M.2.hl.TZ1.4a.i: State the half-equation for the oxidation of the iron(II) ions.
- 09M.2.hl.TZ1.4a.ii: State the half-equation for the reduction of the \({\text{MnO}}_4^ - \) ions in acidic solution.
- 09M.2.hl.TZ1.4a.iii: Deduce the overall redox equation for the reaction.
- 09M.2.hl.TZ1.7a.iii: Deduce which species acts as the oxidizing agent when the cell is operating.
- 09M.2.hl.TZ1.7b.i: \({{\text{[Co(}}{{\text{H}}_{\text{2}}}{\text{O}}{{\text{)}}_{\text{6}}}{\text{]}}^{2 + }}\)
- 09M.2.hl.TZ1.7b.iii: \({{\text{[CoC}}{{\text{l}}_{\text{4}}}{\text{]}}^{2 - }}\)
- 09M.2.hl.TZ1.7b.ii: \({\text{C}}{{\text{o}}_{\text{2}}}{{\text{(S}}{{\text{O}}_{\text{4}}}{\text{)}}_{\text{3}}}\)
- 09M.2.sl.TZ1.4a: Define oxidation in terms of electron transfer.
- 09M.2.sl.TZ1.4b.i: State the oxidation number of manganese in \({\text{KMn}}{{\text{O}}_{\text{4}}}\) and in...
- 09M.2.sl.TZ1.4b.ii: Deduce which species has been oxidized in this reaction and state the change in oxidation...
- 09M.3.sl.TZ1.E2c.i: State what happened to the \({{\text{O}}_{\text{2}}}\) in step 1 in terms of electrons.
- 09M.3.sl.TZ1.D3b.ii: State with a reason whether chromium in potassium dichromate(VI) is oxidised or reduced by...
- 09M.3.sl.TZ1.E2c.ii: State the change in oxidation number for manganese in step 2.
- 09M.3.sl.TZ1.E2a: Outline the meaning of the term biochemical oxygen demand (BOD).
- 09M.1.sl.TZ2.25: Which list represents the halogens in increasing order of oxidizing strength (weakest...
- 09M.1.sl.TZ2.12: Metal M has only one oxidation number and forms a compound with the formula...
- 09M.1.sl.TZ2.24: Which species is oxidized in the following...
- 09M.2.sl.TZ2.5b.i: Define oxidation in terms of oxidation numbers.
- 11M.1.hl.TZ1.36: What is the correct order of reaction types in the following sequence?
- 11M.2.hl.TZ1.9e.i: Deduce the oxidation number of the nitrogen in the reactants and product.
- 11M.2.hl.TZ1.9e.ii: Deduce the oxidation and reduction half-equations and identify the oxidizing agent for the...
- 11M.1.sl.TZ1.23: Which species could be reduced to form \({\text{N}}{{\text{O}}_{\text{2}}}\)? A. ...
- 11M.1.sl.TZ1.25: What happens to the manganese in the following...
- 11M.2.hl.TZ2.7b.ii: Deduce which species can reduce \({\text{S}}{{\text{n}}^{4 + }}{\text{(aq)}}\) to...
- 11M.2.hl.TZ2.7b.iii: Deduce which species can reduce \({\text{S}}{{\text{n}}^{2 + }}{\text{(aq)}}\) to Sn(s) under...
- 11M.2.hl.TZ2.7d.ii: Deduce the oxidation number of chromium in...
- 11M.2.hl.TZ2.7e.i: Explain why it is very difficult to obtain sodium from sodium chloride by any other method.
- 11M.2.hl.TZ2.7b.i: State the species which is the strongest oxidizing agent.
- 11M.2.hl.TZ2.7d.iii: State the balanced half-equation for the reduction of dichromate ions to chromium(III) ions.
- 11M.1.sl.TZ2.24: Consider the following reactions of three unknown metals X, Y and...
- 11M.2.sl.TZ2.2a.i: Deduce the oxidation number of antimony in stibnite.
- 11M.1.sl.TZ2.23: What happens to iodine when iodate ions, \({\text{IO}}_3^ - \), are converted to iodine...
- 11M.2.sl.TZ2.2a.ii: Deduce one other common oxidation number exhibited by antimony in some of its compounds.
- 11M.2.sl.TZ2.2b.i: Deduce the chemical equations for these two reactions.
- 11M.2.sl.TZ2.5b.iii: Deduce the overall equation for the reaction taking place in the voltaic cell and determine...
- 12M.1.sl.TZ2.23: What is the name of \({\text{C}}{{\text{u}}_{\text{2}}}{\text{S}}\)? A. Copper(I)...
- 12M.1.sl.TZ2.24: Consider the following...
- 12M.2.sl.TZ2.1d: (i) Deduce the oxidation numbers of oxygen present in each of the species below. (ii) ...
- 11N.2.hl.TZ0.7a: Distinguish between the terms oxidation and reduction in terms of oxidation numbers.
- 11N.2.hl.TZ0.7b: State the names of \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) and...
- 11N.2.hl.TZ0.7c.i: Define the term oxidizing agent.
- 11N.2.hl.TZ0.7c.ii: \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_7^{2 - }{\text{(aq)}}\) and...
- 11N.1.sl.TZ0.8: Which of the following redox reactions take place? I. ...
- 11N.1.sl.TZ0.23: What is the correct decreasing order of reactivity of the metals X, Y and Z based on the...
- 11N.2.sl.TZ0.5a: Deduce the balanced chemical equation for the reaction between sodium and sulfur. State the...

