| Date | May 2011 | Marks available | 2 | Reference code | 11M.2.sl.TZ2.5 |
| Level | SL | Paper | 2 | Time zone | TZ2 |
| Command term | Deduce | Question number | 5 | Adapted from | N/A |
Question
Ammonia, \({\text{N}}{{\text{H}}_{\text{3}}}\), is a weak base.
Iron is more reactive than copper.
Draw the Lewis structure of ammonia and state the shape of the molecule and its bond angles.
The conjugate acid of ammonia is the ammonium ion, \({\text{NH}}_4^ + \). Draw the Lewis structure of the ammonium ion and deduce its shape and bond angles.
Describe two different properties that could be used to distinguish between a \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of a strong monoprotic acid and a \({\text{1.00 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) solution of a weak monoprotic acid.
Explain, using the Brønsted-Lowry theory, how water can act either as an acid or a base. In each case identify the conjugate acid or base formed.
Draw a labelled diagram of a voltaic cell made from an \({\text{Fe(s)}}/{\text{F}}{{\text{e}}^{2 + }}{\text{(aq)}}\) half-cell connected to a \({\text{Cu(s)}}/{\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}}\) half-cell. In your diagram identify the positive electrode (cathode), the negative electrode (anode) and the direction of electron flow in the external circuit.
Deduce the half-equations for the reactions taking place at the positive electrode (cathode) and negative electrode (anode) of this voltaic cell.
Deduce the overall equation for the reaction taking place in the voltaic cell and determine which species acts as the oxidizing agent and which species has been reduced.
Markscheme
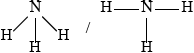 ;
;
Accept any combination of dots/crosses and lines to represent electron pairs.
(trigonal/triangular) pyramid;
Allow 3D representation using wedges and dotted bonds of trigonal pyramidal molecule.
107°;
Accept any angle between 105° and 108.5°.
No ECF for shape based on incorrect Lewis structure.
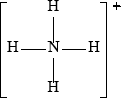 ;
;
Charge needed for mark.
Allow a 3D representation using wedges and dotted bonds of tetrahedral molecule.
109.5°/109°/109° 28';
No ECF for shape based on incorrect Lewis structure.
(measuring) the pH / the strong acid solution will have a lower pH;
conductivity (measurement) / the strong acid will be a better conductor;
the strong acid will react more vigorously with metals/carbonates / the reaction with metals/carbonates;
the heat change when it is neutralized with a base will be different / heat of neutralization / OWTTE;
water can act as a Brønsted-Lowry acid by donating a proton/\({{\text{H}}^ + }\) to form \({\text{O}}{{\text{H}}^ - }\);
water can act as a Brønsted-Lowry base by accepting a proton/\({{\text{H}}^ + }\) to form \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\);
Accept equations showing the above clearly labelling the acid and basic behaviour and the conjugate acid or base.
Award [1 max] for correct definition of how water can act as a Brønsted-Lowry acid or base.
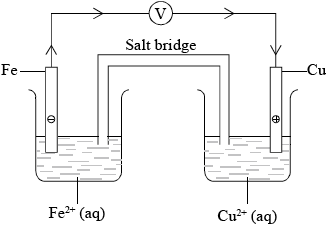
correct diagram including voltmeter/meter, 4 correct species (state symbols not required) and connecting wires;
No credit if wires to electrodes immersed in the solutions.
labelled salt bridge;
Do not accept name of salt (e.g. potassium nitrate) in place of salt bridge.
correctly labelled electrodes (+)/cathode and (–)/anode;
flow of electrons from Fe to Cu in external circuit;
positive electrode: \({\text{C}}{{\text{u}}^{2 + }} + {\text{2}}{{\text{e}}^ - } \to {\text{Cu}}\);
negative electrode: \({\text{Fe}} \to {\text{F}}{{\text{e}}^{2 + }} + {\text{2}}{{\text{e}}^ - }\);
Award [1] if equations correct but at wrong electrodes or if electrodes are missing.
Award [2] for correct equations if electrodes are missing but were correctly labelled in diagram.
Accept e instead of \({e^ - }\).
Ignore state symbols.
Penalize \( \rightleftharpoons \) once only in equations in (ii) and (iii).
\({\text{Fe}} + {\text{C}}{{\text{u}}^{2 + }} \to {\text{F}}{{\text{e}}^{2 + }} + {\text{Cu}}\);
Ignore state symbols.
\({\text{C}}{{\text{u}}^{2 + }}\) is the oxidizing agent and the species that is reduced;
Examiners report
Candidates could draw the Lewis structures in part (a) and generally they could name the shape and suggest the bond angle.
Most knew what a Lewis acid was but some were careless in their definition and said it was an electron acceptor instead of an electron pair acceptor.
Generally candidates could suggest ways of distinguishing between strong and weak acids using pH or conductivity.
The final part of this question caused some difficulty though as students found it hard to show water acting as an acid and a base even though many could correctly state that an acid is a proton donor and a base is a proton acceptor.
Part (b) focused on electrochemistry and although some candidates were able to score 4 marks most lost marks for their diagrams which were often incomplete and/or incorrectly annotated.
Students that could draw the diagram had little problem writing the equations, however many could not do them correctly.
Students that could draw the diagram had little problem writing the equations, however many could not do them correctly. This carried through to the final part of the question and those that could write the half equations could generally write the overall equation. Identifying the oxidizing agent and the species that has been reduced proved tricky as students were reluctant to suggest the same species- \({\text{C}}{{\text{u}}^{2 + }}\), also some students just said copper which was not specific enough to gain the mark.

