| Date | May 2010 | Marks available | 4 | Reference code | 10M.2.sl.TZ1.5 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Deduce and Identify | Question number | 5 | Adapted from | N/A |
Question
Consider the following three redox reactions.
\[\begin{array}{*{20}{l}} {{\text{Cd(s)}} + {\text{N}}{{\text{i}}^{2 + }}{\text{(aq)}} \to {\text{C}}{{\text{d}}^{2 + }}{\text{(aq)}} + {\text{Ni(s)}}} \\ {{\text{Ni(s)}} + {\text{2A}}{{\text{g}}^ + }{\text{(aq)}} \to {\text{N}}{{\text{i}}^{2 + }}{\text{(aq)}} + {\text{2Ag(s)}}} \\ {{\text{Zn(s)}} + {\text{C}}{{\text{d}}^{2 + }}{\text{(aq)}} \to {\text{Z}}{{\text{n}}^{2 + }}{\text{(aq)}} + {\text{Cd(s)}}} \end{array}\]
(i) Draw an annotated diagram of a voltaic cell composed of a magnesium electrode in \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) magnesium nitrate solution and a silver electrode in \({\text{1.0 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}\) silver nitrate solution. State the direction of electron flow on your diagram.
(ii) Deduce half-equations for the oxidation and reduction reactions.
(i) Deduce the order of reactivity of the four metals, cadmium, nickel, silver and zinc and list in order of decreasing reactivity.
(ii) Identify the best oxidizing agent and the best reducing agent.
(i) Solid sodium chloride does not conduct electricity but molten sodium chloride does. Explain this difference.
(ii) Outline what happens in an electrolytic cell during the electrolysis of molten sodium chloride using inert electrodes. Deduce equations for the reactions occurring at each electrode.
(i) A state of equilibrium can exist when a piece of copper metal is placed in a solution of copper(II) sulfate. Outline the characteristics of a chemical system in dynamic equilibrium.
(ii) For an exothermic reaction state how an increase in temperature would affect both \({K_{\text{c}}}\) and the position of equilibrium.
Markscheme
(i) 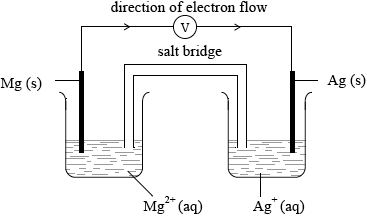
correctly labelled electrodes and solutions;
labelled salt bridge;
voltmeter;
Allow bulb or ammeter.
direction of electron flow;
(ii) Oxidation:
\({\text{Mg(s)}} \to {\text{M}}{{\text{g}}^{2 + }}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\);
Reduction:
\({\text{A}}{{\text{g}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \to {\text{Ag(s)}}\);
Ignore state symbols.
Award [1 max] if equations not labelled reduction or oxidation or labelled the wrong way round.
Allow e instead of e–.
Penalize equilibrium sign or reversible arrows once only in parts (a) (ii) and (c) (ii).
(i) \({\text{Zn}} > {\text{Cd}} > {\text{Ni}} > {\text{Ag}}\)
Zn most reactive;
rest of order correct;
(ii) Best oxidizing agent:
\({\text{A}}{{\text{g}}^ + }\);
Do not accept Ag.
Best reducing agent:
Zn;
Do not accept Zn2+.
(i) sodium chloride crystals consist of ions in a (rigid) lattice / ions cannot move (to electrodes) / OWTTE;
when melted ions free to move / ions move when potential difference/voltage applied;
(ii) positive sodium ions/\({\text{N}}{{\text{a}}^ + }\) move to negative electrode/cathode and negative chloride ions/ \({\text{C}}{{\text{l}}^ - }\) move to positive electrode/anode;
electrons released to positive electrode/anode by negative ions and accepted from negative electrode/cathode by positive ions / reduction occurs at the negative electrode/cathode and oxidation occurs at the positive electrode/anode / \({\text{N}}{{\text{a}}^ + }\) ions are reduced and \({\text{C}}{{\text{l}}^ - }\) ions are oxidized;
(Positive electrode/anode):
\({\text{2C}}{{\text{l}}^ - } \to {\text{C}}{{\text{l}}_2} + {\text{2}}{{\text{e}}^ - }{\text{ / C}}{{\text{l}}^ - } \to \frac{1}{2}{\text{C}}{{\text{l}}_2} + {{\text{e}}^ - }\);
(Negative electrode/cathode):
\({\text{2N}}{{\text{a}}^ + } + {\text{2}}{{\text{e}}^ - } \to {\text{2Na / N}}{{\text{a}}^ + } + {{\text{e}}^ - } \to {\text{Na}}\);
Award [1 max] if equations not labelled or labelled wrong way round.
Allow e instead of e–.
Penalize equilibrium sign or reversible arrows once only in parts (a) (ii) and (c) (ii).
(i) macroscopic properties remain constant / concentrations remain constant / no change to copper solution seen;
rate of reverse/backwards reaction = rate of forward reaction;
(ii) \({K_{\text{c}}}\) decreases;
position of equilibrium shifts to left;
Examiners report
This question was also chosen by approximately 40% of candidates. In (a), the diagram was reasonably attempted by most candidates, with just a few candidates giving both electrodes in one beaker. Some candidates omitted to include a voltmeter and other common mistakes included omission of states and incorrect direction of electron flow. In (ii), fewer candidates scored these marks and many equations were not labelled explicitly as oxidation and reduction. Other common errors included incorrect charges for the silver and magnesium ions.
In (b), most candidates were able to place the metals in order, though a small minority misread the question, and gave zinc as the least reactive. In (ii), zinc was generally given as the best reducing agent, but often silver metal rather than silver(I) ion as given as the best oxidizing agent.
There were many references to sodium chloride having a metallic structure in (c) (i), and describing its conduction in terms of electrons rather than ions. In (ii), very few candidates mentioned that the ions move towards the oppositely charged electrode. The nature of the electrolytic process was not well explained.
The characteristics of a chemical system in a dynamic equilibrium in (d) (i) typically were understood by most candidates, although many just scored one mark. (ii) was well answered.

