| Date | May 2013 | Marks available | 2 | Reference code | 13M.2.hl.TZ2.7 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Define | Question number | 7 | Adapted from | N/A |
Question
A voltaic cell was set up, using the standard hydrogen electrode as a reference electrode and a standard \({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)/Cu(s)}}\) electrode.
Another voltaic cell was set up, using a \({\text{S}}{{\text{n}}^{2 + }}{\text{(aq)/Sn(s)}}\) half-cell and a \({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)/Cu(s)}}\) half-cell under standard conditions.
Water in a beaker at a pressure of \(1.01 \times {10^5}{\text{ Pa}}\) and a temperature of 298 K will not spontaneously decompose. However, decomposition of water can be induced by means of electrolysis.
Define oxidation in terms of oxidation number.
Deduce the balanced chemical equation for the redox reaction of copper, Cu(s), with nitrate ions, \({\text{N}}{{\text{O}}^{3 - }}{\text{(aq)}}\), in acid, to produce copper(II) ions, \({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}}\), and nitrogen(IV) oxide, \({\text{N}}{{\text{O}}_{\text{2}}}{\text{(g)}}\).
Deduce the oxidizing and reducing agents in this reaction.
Oxidizing agent:
Reducing agent:
Describe the standard hydrogen electrode including a fully labelled diagram.
Define the term standard electrode potential, \({E^\Theta }\).
Deduce a balanced chemical equation, including state symbols, for the overall reaction which will occur spontaneously when the two half-cells are connected.
Draw a fully labelled diagram of the voltaic cell, showing the positive electrode (cathode), the negative electrode (anode) and the direction of electron movement through the external circuit.
Using Table 14 of the Data Booklet, calculate the cell potential, \(E_{{\text{cell}}}^\Theta \), in V, when the two half-cells are connected.
Deduce the sign of the standard free energy change, \(\Delta {G^\Theta }\), for any non-spontaneous reaction.
State why dilute sulfuric acid needs to be added in order for the current to flow in the electrolytic cell.
State why copper electrodes cannot be used in the electrolysis of water. Suggest instead suitable metallic electrodes for this electrolysis process.
Deduce the half-equations for the reactions occurring at the positive electrode (anode) and the negative electrode (cathode).
Positive electrode (anode):
Negative electrode (cathode):
Deduce the overall cell reaction, including state symbols.
Draw a fully labelled diagram of the electrolytic cell, showing the positive electrode (anode) and the negative electrode (cathode).
Comment on what is observed at both electrodes.
Two electrolytic cells are connected in series (the same current passes through each cell). One cell for the electrolysis of water produces 100 cm\(^3\) of oxygen, measured at 273 K and \(1.01 \times {10^5}{\text{ Pa}}\). The second cell contains molten lead(II) bromide, \({\text{PbB}}{{\text{r}}_{\text{2}}}\). Determine the mass, in g, of lead produced.
Markscheme
increase (in oxidation number);
\({\text{Cu(s)}} + {\text{2NO}}_3^ - {\text{(aq)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} + {\text{2N}}{{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(l) /}}\)
\({\text{Cu(s)}} + {\text{2HN}}{{\text{O}}_3}{\text{(aq)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{C}}{{\text{u}}^{2 + }} + {\text{2N}}{{\text{O}}_2}{\text{(g)}} + {\text{2HO(l)}}\);
correct reactants and products;
fully balanced chemical equation;
Ignore state symbols.
M1 can be scored if there are unbalanced electrons in equation.
M2 can only be scored if M1 is correct.
M2 can be scored if there are balanced electrons on both sides of equation.
Oxidizing agent: \({\text{NO}}_{\text{3}}^ - \)/nitrate/\({\text{HN}}{{\text{O}}_{\text{3}}}\)/nitric acid and Reducing agent: Cu/copper;
Diagram showing gas, solution and solid electrode;
For example,
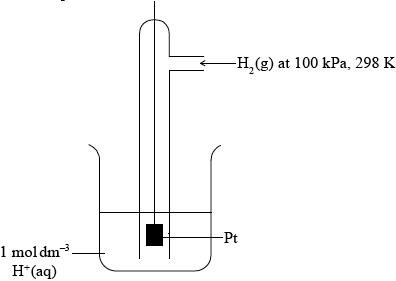
\({\text{1 mol}}\,{\text{d}}{{\text{m}}^{ - 3}}{\text{ }}{{\text{H}}^ + }\) (aq) and Pt;
Allow 1 mol L–1 or 1 M.
Allow 1 mol dm–3 HCl (aq) or other source of 1mol dm−3 H+(aq) ions.
100 kPa/10\(^5\) Pa/1 bar (H\(_2\) (g) pressure) and 298K / 25 °C;
Ignore state symbols throughout.
Allow 1.01×10\(^5\) Pa/1 atm.
potential of reduction half-reaction under standard conditions measured relative to standard hydrogen electrode/SHE / potential under standard conditions relative to standard hydrogen electrode/SHE;
Instead of standard conditions allow either solute concentration of 1 mol\(\,\)dm–3 or 100 kPa/105 Pa/1 bar (pressure) for gases (allow 1 atm).
Allow voltage/EMF instead of potential.
\({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}} + {{\text{H}}_2}{\text{(g)}} \to {\text{Cu(s)}} + {\text{2}}{{\text{H}}^ + }{\text{(aq)}}\)
reactants and products;
fully balanced chemical equation, including state symbols;
M2 cannot be scored if M1 is incorrect.
Voltaic cell showing:
Labelled positive electrode (cathode): Cu2+ /Cu and negative electrode (anode): Sn2+ /Sn;
Do not penalize if state symbols are not included (since given in question).
voltmeter and salt bridge;
Voltmeter can be labelled or drawn as a circle with a V.
Allow ammeter/A.
Salt bridge can be labelled, represented with drawing connecting the two half-cells, labelled as potassium nitrate or using its chemical formula (for example, KNO3) etc.
correct direction of electron movement from Sn to Cu in external circuit;
\(( + )0.48{\text{ (V)}}\);
positive;
provides ions (to carry current) / water poor conductor (of electricity);
Do not accept electrons instead of ions.
copper reacts so (nonreactive metal such as) Pt used;
Accept Ag, Au or any named metal less reactive than copper as electrode.
Do not accept Cu reacts with water or graphite as electrode.
Positive electrode (anode):
\({\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {{\text{O}}_2}{\text{(g)}} + {\text{4}}{{\text{H}}^ + }{\text{(aq)}} + {\text{4}}{{\text{e}}^ - }{\text{ / 4O}}{{\text{H}}^ - }{\text{(aq)}} \to {{\text{O}}_2}{\text{(g)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{4}}{{\text{e}}^ - }\);
Negative electrode (cathode):
\({{\text{H}}^ + }{\text{(aq)}} + {{\text{e}}^ - } \to \frac{1}{2}{{\text{H}}_2}{\text{(g) / 4}}{{\text{H}}^ + }{\text{(aq)}} + {\text{4}}{{\text{e}}^ - } \to {\text{2}}{{\text{H}}_2}{\text{(g) / 2}}{{\text{H}}^ + }{\text{(aq)}} + {\text{2}}{{\text{e}}^ - } \to {{\text{H}}_2}{\text{ /}}\)
\({\text{2}}{{\text{H}}_2}{\text{O(l)}} + {\text{2}}{{\text{e}}^ - } \to {{\text{H}}_2}{\text{(g)}} + {\text{2O}}{{\text{H}}^ - }{\text{(aq) / }}{{\text{H}}_2}{\text{O(l)}} + {{\text{e}}^ - } \to \frac{1}{2}{{\text{H}}_2}{\text{(g)}} + {\text{O}}{{\text{H}}^ - }\);
Award [1 max] if M1 and M2 reversed.
Ignore state symbols.
Allow e instead of e–.
Do not penalize use of equilibrium sign instead of \( \to \).
Accept a multiple of the equations.
\({\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {\text{2}}{{\text{H}}_2}{\text{(g)}} + {{\text{O}}_2}{\text{(g) / }}{{\text{H}}_2}{\text{O(l)}} \to {{\text{H}}_2}{\text{(g)}} + \frac{1}{2}{{\text{O}}_2}{\text{(g)}}\);
State symbols required as asked for in question.
Do not penalize use of equilibrium sign instead of \( \to \).
Do not accept any multiple of 2H+(aq) + 2OH–(aq) \( \to \) 2H2(g) + O2(g).
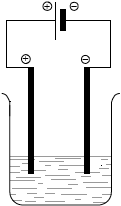
electrolytic cell showing solid electrodes immersed in solution;
positive electrode (anode) connected to positive terminal of battery and
negative electrode (cathode) to negative terminal;
Allow graphite or metal given in e(iii) as electrodes.
bubbles /gas produced;
Do not accept hydrogen is formed at cathode and oxygen formed at anode.
\(n({{\text{O}}_2}){\text{ }}\left( { = \left( {\frac{{100}}{{22.4 \times 1000}}} \right)} \right) = 4.46 \times {10^{ - 3}}{\text{ (mol)}}\);
\(m{\text{ }}\left( { = (4.46 \times {{10}^{ - 3}} \times 2 \times 207.19)} \right) = 1.85{\text{ (g)}}\);
OR
\(n({{\text{O}}_2}){\text{ }}\left( { = \frac{{{\text{PV}}}}{{{\text{RT}}}}} \right) = 4.45 \times {10^{ - 3}}{\text{ (mol)}}\);
\(m{\text{ }}( = 4.45 \times {10^{ - 3}} \times 2 \times 207.19) = 1.84{\text{ (g)}}\);
Examiners report
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.
Many made mistakes in writing a balanced equation for the reaction between Cu and HNO3, in drawing a diagram for a hydrogen electrode, in writing a definition of ‘standard electrode potential’. Most could draw a labeled diagram for an electrochemical cell. Many mistakes were made in writing balanced equations for reactions at the electrodes and overall equation for the electrolysis of water.

