| Date | May 2015 | Marks available | 1 | Reference code | 15M.2.hl.TZ2.8 |
| Level | HL | Paper | 2 | Time zone | TZ2 |
| Command term | Deduce | Question number | 8 | Adapted from | N/A |
Question
Chromium is a transition metal with many uses.
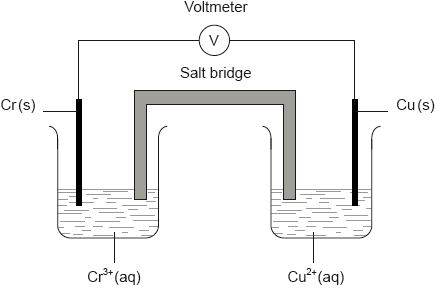
A voltaic cell is constructed as follows. One half-cell contains a chromium electrode immersed in a solution containing \({\text{C}}{{\text{r}}^{3 + }}{\text{(aq)}}\) ions. The other half-cell contains a copper electrode immersed in a solution containing \({\text{C}}{{\text{u}}^{2 + }}{\text{(aq)}}\) ions. The two electrodes are connected to a voltmeter and the two solutions by a salt bridge.
Draw an orbital diagram (using the arrow-in-box notation) showing the electrons in the 4s and 3d sub-levels in chromium metal.
Outline the nature of the metallic bonding present in chromium.
Explain why chromium metal is malleable.
State the name of \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\).
Describe the ionic bonding present in \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) and how the ions are formed.
Suggest why solid \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) does not conduct electricity.
Chromium forms the complex ion \({[{\text{Cr}}{({\text{N}}{{\text{H}}_{\text{3}}})_{\text{4}}}{\text{C}}{{\text{l}}_2}]^ + }\).
Deduce the oxidation number of chromium in this complex.
Chromium forms the complex ion \({[{\text{Cr}}{({\text{N}}{{\text{H}}_{\text{3}}})_{\text{4}}}{\text{C}}{{\text{l}}_2}]^ + }\).
Describe the nature of the ligand-chromium ion bonds in terms of acid-base theory.
Chromium forms the complex ion \({[{\text{Cr}}{({\text{N}}{{\text{H}}_{\text{3}}})_{\text{4}}}{\text{C}}{{\text{l}}_2}]^ + }\).
Explain why \({[{\text{Cr}}{({\text{N}}{{\text{H}}_{\text{3}}})_{\text{4}}}{\text{C}}{{\text{l}}_{\text{2}}}{\text{]}}^ + }\) is coloured.
Chromium forms the complex ion \({[{\text{Cr}}{({\text{N}}{{\text{H}}_{\text{3}}})_{\text{4}}}{\text{C}}{{\text{l}}_2}]^ + }\).
Draw the structures of two possible isomers of this complex ion.
The dichromate ion, \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{2 - }{\text{(aq)}}\), and the iodide ion, \({{\text{I}}^ - }{\text{(aq)}}\), react together in the presence of an acid to form \({\text{C}}{{\text{r}}^{3 + }}{\text{(aq)}}\) and \({\text{IO}}_3^ - {\text{(aq)}}\) ions. Deduce the half-equation for the reaction of \({{\text{I}}^ - }\) to \({\text{IO}}_3^ - \) and the overall equation for this reaction.
Half-equation:
Overall equation:
Explain in terms of oxidation numbers whether iodine is oxidized or reduced in part (d) (i).
Define the term standard electrode potential.
Calculate the cell potential, in V, under standard conditions, for this voltaic cell, using table 14 of the data booklet and \({\text{E}}_{{\text{C}}{{\text{r}}^{3 + }}/{\text{Cr}}}^\Theta = -0.74{\text{ V}}\).
Predict the balanced equation for the spontaneous reaction which will produce a current in this voltaic cell.
Identify the negative and the positive electrodes in this cell.
Predict the direction of movement of electrons in the external circuit.
State the directions in which the negative ions (anions) and the positive ions (cations) flow in the salt bridge.
Markscheme
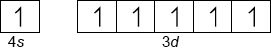
Accept full-arrows.
Accept boxes in reverse order or at different energy levels.
Do not award the mark if sub-levels are incorrectly labelled or if no boxes are drawn.
(electrostatic) attraction between (lattice of) cations/positive/\({\text{C}}{{\text{r}}^{3 + }}\) ions and delocalized electrons;
(delocalized electrons allows) the layers/rows of cations/positive/\({\text{C}}{{\text{r}}^{3 + }}\) ions to slide past each other (without disrupting the metallic bonding);
Accept atoms instead of ions.
chromium(III) oxide;
Do not award the mark for chromium oxide.
(electrostatic) attraction between positive and negative ions/oppositely charged ions/\({\text{C}}{{\text{r}}^{3 + }}\) and \({{\text{O}}^{2 - }}\);
formed as a result of electron transfer from chromium atoms to oxygen atoms / OWTTE;
Ignore reference to number of electrons transferred or charges of ion for M2.
ions are not free to move (when solid) / ions in rigid lattice / OWTTE;
III / +3;
Do not accept incorrect notation such as 3+/3.
ligand has lone/non-bonding electron pair /
dative (covalent)/coordinate/coordination bond forms;
ligand is Lewis base / ion is Lewis acid;
partially filled/incomplete d sub levels/orbitals;
d orbitals split into two levels;
energy difference is in visible part of spectrum / electrons absorb visible light/one colour/frequency/wavelength;
electron transitions occur from lower to higher energy level (within d sub-level);
complementary colour/colour not absorbed is seen;
Do not accept complementary colour "emitted".
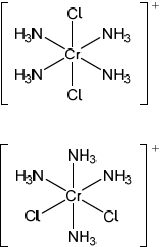 ;
;
Accept any other octahedral arrangement of ligands.
Ignore missing square brackets and charge.
Half equation:
\({{\text{I}}^ - }{\text{(aq)}} + {\text{3}}{{\text{H}}_2}{\text{O(l)}} \to {\text{IO}}_3^ - {\text{(aq)}} + {\text{6}}{{\text{H}}^ + }{\text{(aq)}} + {\text{6}}{{\text{e}}^ - }\);
Accept e instead of \({e^ - }\).
Accept reversible arrows.
Overall equation:
\({\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {{\text{I}}^ - }{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{IO}}_3^ - {\text{(aq)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\);
Ignore state symbols.
oxidized and increase (in oxidation number) of 6/from –1/–I to +5/+V;
potential (of reduction half-reaction) under standard conditions measured relative to standard hydrogen electrode/SHE / OWTTE;
Accept “solute concentration of 1 \(mol\,d{m^{ - 3}}\)” or “1 bar/1 atm (pressure) for gases” instead of “standard conditions”.
Accept voltage/emf for potential.
\(( + )1.08{\text{ (V)}}\);
\({\text{2Cr(s)}} + {\text{3C}}{{\text{u}}^{2 + }}{\text{(aq)}} \to {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{3Cu(s)}}\);
Ignore state symbols.
Do not accept reversible arrows.
Negative electrode: chromium/Cr and Positive electrode: copper/Cu;
Accept “Cr is the anode and Cu the cathode”.
from chromium/Cr to copper/Cu;
Accept “from negative electrode/anode to positive electrode/cathode” if electrodes correctly identified in (iv).
negative ions/anions towards the chromium(III) solution and positive ions/cations towards the copper(II) solution / OWTTE;
Examiners report
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.
Most candidates were able to draw an arrow in the box diagram for the electron configuration of chromium, but few gave a complete description of the nature of metallic bonding and did not refer to the attraction between the \({\text{C}}{{\text{r}}^{3 + }}\) cations and the delocalized electrons. Candidates were more successful in explaining malleability in terms of \({\text{C}}{{\text{r}}^{3 + }}\) cations sliding over one another. Most candidates were able to use oxidation numbers in naming \({\text{C}}{{\text{r}}_{\text{2}}}{{\text{O}}_{\text{3}}}\) but the explanation of ionic bonding was incomplete with only limited reference to the electrostatic attraction between the oppositely charged ions. Students continue to struggle to understand that conductivity of molten ionic compounds is due to mobile ions not electrons. Most candidates were able to deduce the oxidation number in the complex ion and give the answer using the correct notation. The nature of the ligand-chromium bond was well known and the explanation of the colour of transition metal complexes was stronger than in previous sessions with only a minority referring to the emission of light. Some teachers have commented that trans/cis-isomers of the complex ions is not specifically stated in the guide but many students were able draw two possible isomers. The representation of 3D structures could have been clearer although this was not explicitly penalized. Redox half-reactions continue to challenge many with only the stronger students being able to gain both marks and deduce the correct overall equation. Some teachers commented that the question was too demanding as students had to construct two half-equations in order to get to the overall redox equation, but the \({\text{C}}{{\text{r}}_{\text{2}}}{\text{O}}_{\text{7}}^{{\text{2}} - }{\text{/C}}{{\text{r}}^{3 + }}\) half-reaction is given in table 14 of the current data booklet. The majority of candidates identified the conversion of \({{\text{I}}^ - }\) to \({\text{I}}{{\text{O}}^{3 - }}\) as oxidation and many able to identify the increase in oxidation number. The workings of a voltaic cell was generally well understood but the definition of the term standard electrode potential was often incomplete with the reference to standard conditions of the hydrogen electrode often missing.

