| Date | May 2009 | Marks available | 1 | Reference code | 09M.2.sl.TZ1.5 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Explain | Question number | 5 | Adapted from | N/A |
Question
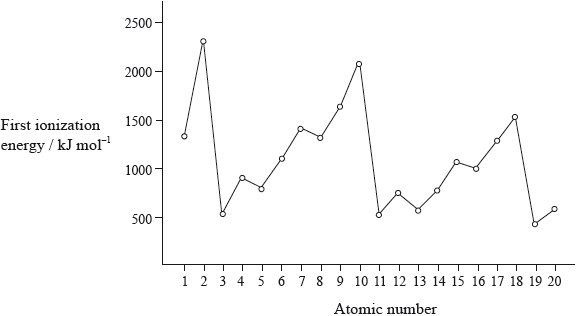
The graph of the first ionization energy plotted against atomic number for the first twenty elements shows periodicity.
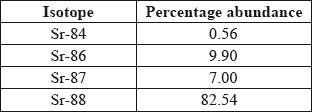
Strontium exists as four naturally-occurring isotopes. Calculate the relative atomic mass of strontium to two decimal places from the following data.
Define the term first ionization energy and state what is meant by the term periodicity.
State the electron arrangement of argon and explain why the noble gases, helium, neon and argon show the highest first ionization energies for their respective periods.
A graph of atomic radius plotted against atomic number shows that the atomic radius decreases across a period. Explain why chlorine has a smaller atomic radius than sodium.
Explain why a sulfide ion, \({{\text{S}}^{2 - }}\), is larger than a chloride ion, \({\text{C}}{{\text{l}}^ - }\).
Explain why the melting points of the Group 1 metals \({\text{(Li}} \to {\text{Cs)}}\) decrease down the group whereas the melting points of the Group 7 elements \({\text{(F}} \to {\text{I)}}\) increase down the group.
Markscheme
\({A_{\text{r}}} = \frac{{[(0.56 \times 84) + (9.90 \times 86) + (7.00 \times 87) + (82.54 \times 88)]}}{{100}}\);
\( = 87.71\);
Award [1 max] if answer not given to two decimal places.
Award [2] for correct final answer.
Apply –1(U) if answer quoted in g or g\(\,\)mol–1.
first ionization energy: \({\text{M(g)}} \to {{\text{M}}^ + }{\text{(g)}} + {{\text{e}}^ - }{\text{/e}}\) / the (minimum) energy (\({\text{in kJ}}\,{\text{mo}}{{\text{l}}^{ - 1}}\)) to remove one electron from a gaseous atom / the energy required to remove one mole of electrons from one mole of gaseous atoms;
periodicity: repeating pattern of (physical and chemical) properties;
2.8.8/sp version;
Accept any two of the following:
the outer energy level/shell is full;
the increased charge on the nucleus;
great(est) attraction for electrons;
17 p in Cl nucleus attract the outer level more than 11 p in Na nucleus / greater nuclear charge attracts outer level more;
Allow converse for Na.
Do not accept larger nucleus.
\({{\text{S}}^{2 - }}\) has one proton less/ smaller nuclear charge so outer level held less strongly / OWTTE;
Allow converse for chloride.
Do not accept larger nucleus.
the radii of the metal atoms increase (from \({\text{Li}} \to {\text{Cs}}\)) (so the forces of attraction are less between them) / OWTTE;
the forces of attraction between halogen molecules are van der Waals;
forces increase with increasing mass/number of electrons;
Examiners report
The type of calculation in (a)(iii)is well understood. In the cases where both marks were not awarded, this was due to arithmetic error, not reading the question properly and providing an answer to other than two decimal places, or giving some unit such as grams.
Part (b)(i) resulted in very few marks being awarded; the requirements to refer to a gaseous atom and the idea of repetition in periodicity were sufficient to prevent otherwise reasonable answers from scoring.
The electron configuration was usually known in (b)(ii), as was the fact that there is a full outer electron shell. The third mark was less frequently awarded, with candidates often using the simplistic ‘it is full so it doesn’t want to lose electrons’ argument.
(b)(iii) was commonly correct but (b)(iv) was less well answered as many candidates failed to realise that these ions are isoelectronic and gave an answer relating to sulphide having more electrons with a consequent increase in repulsions.
(b)(iii) was commonly correct but (b)(iv) was less well answered as many candidates failed to realise that these ions are isoelectronic and gave an answer relating to sulphide having more electrons with a consequent increase in repulsions.
Part (b)(v) was poorly answered. Very few scored the first mark, many answers referring to some sort of ionization process. A handful of candidates scored the second mark for a reference to van der Waals forces but explanations for an increase were very weak. A number of candidates lost marks by referring to the breaking of covalent bonds rather than overcoming intermolecular forces.

