| Date | November 2011 | Marks available | 4 | Reference code | 11N.2.sl.TZ0.5 |
| Level | SL | Paper | 2 | Time zone | TZ0 |
| Command term | Describe and State | Question number | 5 | Adapted from | N/A |
Question
Phosphorus tribromide (\({\text{PB}}{{\text{r}}_{\text{3}}}\)) is used to manufacture alprazolam, a drug used to treat anxiety disorders. Methanal (HCHO) is used as a disinfectant.
Consider the following reaction sequence:
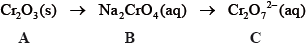
Deduce the balanced chemical equation for the reaction between sodium and sulfur. State the electron arrangements of the reactants and product, and explain whether sulfur is oxidized or reduced.
Describe the acid-base character of the oxides of the period 3 elements, Na to Cl. For the compounds sodium oxide and phosphorus(V) oxide, state the balanced chemical equations for the reaction of each oxide with water.
For each of the species \({\text{PB}}{{\text{r}}_{\text{3}}}\) and HCHO:
• deduce the Lewis structure.
• predict the shape and bond angle.
Explain why \({\text{PB}}{{\text{r}}_{\text{3}}}\) is a polar molecule.
State the name of A.
Describe the redox behaviour of chromium with reference to oxidation numbers in the conversion of B to C.
Define the term oxidizing agent and identify the oxidizing agent in the following
reaction.
\[{\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }{\text{(aq)}} + {{\text{I}}^ - }{\text{(aq)}} + {\text{8}}{{\text{H}}^ + }{\text{(aq)}} \to {\text{2C}}{{\text{r}}^{3 + }}{\text{(aq)}} + {\text{IO}}_3^ - {\text{(aq)}} + {\text{4}}{{\text{H}}_2}{\text{O(l)}}\]
Markscheme
\({\text{2Na(s)}} + {\text{S(s)}} \to {\text{N}}{{\text{a}}_2}{\text{S(s)}}/{\text{2Na(s)}} + \frac{1}{2}{{\text{S}}_2}{\text{(g)}} \to {\text{N}}{{\text{a}}_2}{\text{S(s)}}/{\text{16Na(s)}} + {{\text{S}}_2}{\text{(s)}} \to {\text{8N}}{{\text{a}}_2}{\text{S(s)}}\);
Ignore state symbols.
Na: 2, 8,1 and S: 2, 8, 6;
\({\text{N}}{{\text{a}}^ + }\): 2, 8 and \({{\text{S}}^{2 - }}\): 2,8,8;
reduced since it has gained electrons / reduced since oxidation number has decreased;
Do not award mark if incorrect oxidation numbers are given.
Na, Mg: basic
Al: amphoteric
Do not accept amphiprotic.
Si to Cl: acidic
Award [2] for all three listed sets correct, [1] for one or two listed sets correct.
Award [1] for stating oxides become more basic towards left/Na and more acidic towards right/Cl.
Do not penalize incorrect formulas of oxides.
\({\text{N}}{{\text{a}}_2}{\text{O(s)}} + {{\text{H}}_2}{\text{O(l)}} \to {\text{2NaOH(aq)}}\);
\({{\text{P}}_4}{{\text{O}}_{10}}{\text{(s)}} + {\text{6}}{{\text{H}}_2}{\text{O(l)}} \to {\text{4}}{{\text{H}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}}\);
Ignore state symbols.
Allow P2O3(s) \( + \) 3H2O(l) \( \to \) 2H3PO4(aq).
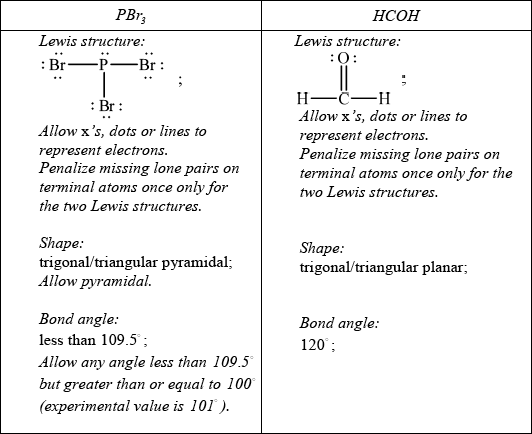
Do not allow ECF in this question from incorrect Lewis structure.
Br more electronegative than P / P–Br bond polar;
bond dipoles do not cancel / there is a net dipole / asymmetric distribution of electron cloud;
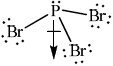
Allow polar bonds do not cancel or that it is an asymmetric molecule.
Award [2] for diagram showing net dipole moment as shown.
chromium(III) oxide;
Do not award mark for chromium oxide.
chromium is neither oxidized or reduced since there is no change in oxidation number/+6 to +6;
substance reduced / causes other substance to be oxidized / increase oxidation number of another species / gains electrons / OWTTE;
Oxidizing agent:
\({\text{C}}{{\text{r}}_2}{\text{O}}_7^{2 - }\) / dichromate (ion);
Examiners report
This was one of the least popular questions but those candidates that did attempt it, often performed well. In part (a) the equation was well answered, as were the electron arrangements of sodium and sulfur, but candidates struggled with the electron arrangements of the ions. Also, some forgot to give a reason as to why sulfur is reduced.
The first part on acid-base behaviour was well answered though a few stated that silicon is amphoteric which is incorrect, unfortunately this is an error that has appeared in some IB textbooks. As regards the equations, hydrogen was often given as a product, and although many could successfully write the equation of sodium oxide with water, very few could successfully write the equation with phosphorous (V) oxide.
In part (c) candidates could draw the Lewis structures and generally they could name the shape and suggest the bond angle. However lone pairs were often omitted, especially on oxygen and bromine.
Explaining molecular polarity often was more challenging, and clearly it is poorly understood.
Few candidates correctly used the Roman numeral III.
Many candidates did realise in part (ii) that there was no change in oxidation number of chromium and so no redox reaction.
In part (iii) candidates could define what an oxidizing agent was and most correctly identified dichromate, as the oxidizing agent, however some just incorrectly stated chromium.

