| Date | May 2014 | Marks available | 1 | Reference code | 14M.1.sl.TZ1.9 |
| Level | SL | Paper | 1 | Time zone | TZ1 |
| Command term | Question number | 9 | Adapted from | N/A |
Question
The electronegativities of four elements are given in the table.
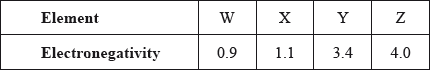
Which statement is correct?
A. W and X form an ionic compound.
B. W and X form a covalent compound.
C. Y and Z form an ionic compound.
D. Y and Z form a covalent compound.
Markscheme
D
Examiners report
Some teachers commented that two answers were acceptable as the difference of electronegativity between W and X and between Y and Z were both small, which could indicate a covalent bond. Although the students did not have access to the data booklet, the values of electronegativity of W and X were much smaller than those for Y and Z, indicating that they were metals. However, in fairness to the candidates it was decided to accept both answers B and D.
Syllabus sections
Show 143 related questions
- 17N.2.sl.TZ0.2c: State an equation for the reaction of phosphorus (V) oxide, P4O10 (s), with water.
- 17N.2.sl.TZ0.2b: Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group.
- 17N.2.sl.TZ0.2a: Explain the general increasing trend in the first ionization energies of the period...
- 17N.1.sl.TZ0.8: Which oxide dissolves in water to give a solution with a pH below 7? A. MgO B. Li2O C....
- 17N.1.sl.TZ0.7: Which trends are correct across period 3 (from Na to Cl)? I. Atomic radius decreasesII. ...
- 17N.2.hl.TZ0.3b: Explain why the melting points of the group 1 metals (Li → Cs) decrease down the group...
- 17M.2.sl.TZ2.1c.i: Some oxides of period 3, such as Na2O and P4O10, react with water. A spatula measure of each...
- 17M.1.hl.TZ2.5: X, Y and Z represent the successive elements, Ne, Na and Mg, but not necessarily in that...
- 17M.1.sl.TZ2.12: Which metal has the strongest metallic bond? A. Li B. Na C. K D. Rb
- 17M.2.hl.TZ1.2d.ii: Suggest why the melting point of vanadium is higher than that of titanium.
- 17M.2.sl.TZ1.2d.ii: Explain why an aluminium-titanium alloy is harder than pure aluminium.
- 17M.1.hl.TZ1.6: What is the order of decreasing ionic radius? A. S2− > Cl− > Al3+ > Mg2+ B. ...
- 17M.1.sl.TZ1.8: Which oxide, when added to water, produces the solution with the highest pH? A. Na2O B....
- 16N.2.sl.TZ0.4e: In addition to magnesium oxide, magnesium forms another compound when burned in air. Suggest...
- 16N.2.sl.TZ0.4d: Describe the trend in acid-base properties of the oxides of period 3, sodium to chlorine.
- 16N.1.hl.TZ0.8: Which correctly describes the reaction between potassium and excess water? A. The reaction...
- 16N.1.hl.TZ0.7: Which property increases down group 17, the halogens? A. Electron affinity B. Boiling...
- 16N.1.sl.TZ0.8: Which solution forms when phosphorus(V) oxide, P4O10, reacts with water?
- 16N.1.sl.TZ0.7: Which equation represents the first electron affinity of chlorine? A. Cl(g)+e-→ Cl-(g)B....
- 16M.2.hl.TZ0.1d: Impurities cause phosphine to ignite spontaneously in air to form an oxide of phosphorus and...
- 16M.2.sl.TZ0.2b: (i) State the equation for the reaction of this oxide of phosphorus with water. (ii) Predict...
- 16M.1.sl.TZ0.8: Which periodic trend is described correctly?
- 16M.1.sl.TZ0.7: Which element is a metalloid? A. Co B. As C. Cs D. Es
- 15M.1.hl.TZ2.7: What is the definition of electronegativity? A. The relative measure of the tendency of...
- 15M.2.hl.TZ1.2a: State the equations for the reactions of sodium oxide with water and phosphorus(V) oxide with...
- 15M.2.hl.TZ2.9a.ii: Suggest, giving your reasons, the approximate pH values of the solutions formed by adding...
- 15M.2.hl.TZ2.9b.i: Identify the acid-base character of the oxides of each of the elements from sodium to...
- 15M.2.hl.TZ2.9b.ii: State the equations for the separate reactions of sodium oxide and phosphorus(V) oxide with...
- 15M.1.sl.TZ2.7: Which statement is correct for the halogens \({\text{(F}} \to {\text{I)}}\)? A. ...
- 15M.1.sl.TZ2.8: Which combination of properties best describes sodium oxide,...
- 15M.2.sl.TZ1.6a: Define the term electronegativity.
- 15M.2.sl.TZ1.6b: Explain why the atomic radius of elements decreases across the period.
- 15M.2.sl.TZ1.6c.i: State the equations for the reactions of sodium oxide with water and phosphorus(V) oxide with...
- 15M.2.sl.TZ2.6b.i: State the equation for the reaction between potassium and chlorine.
- 15M.2.sl.TZ2.6c.i: Identify the acid-base character of the oxides of each of the elements from sodium to...
- 15M.2.sl.TZ2.6c.ii: State the equations for the separate reactions of sodium oxide and phosphorus(V) oxide with...
- 14M.1.hl.TZ1.7: Which statements about reactivity are correct? I. Potassium reacts more vigorously than...
- 14M.2.hl.TZ1.5a: (i) State the changes in the acid-base nature of the oxides across period 3 (from...
- 14M.2.hl.TZ1.6b: (i) Deduce the order of reactivity of these four metals, from the least to the most...
- 14M.2.hl.TZ2.5a: (i) Describe the colour change that occurs when aqueous chlorine is added to aqueous...
- 14M.2.hl.TZ1.5b: (i) Predict any changes that may be observed in each...
- 14M.2.hl.TZ2.8a: Define the term first ionization energy.
- 15M.1.sl.TZ1.8: What is the definition of the term first ionization energy? A. The energy released when...
- 14M.1.sl.TZ1.8: The horizontal axis of the bar chart represents the elements of period 3 from sodium to...
- 14M.2.sl.TZ1.5a: (i) State the equation for the reaction of sodium metal with water. (ii) Describe...
- 14M.2.sl.TZ1.5e.i: \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) does not conduct electricity in the solid...
- 14M.2.sl.TZ1.5e.iii: State equations for the reactions of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and...
- 14M.1.sl.TZ2.7: Which properties decrease down group 1? I. Melting point II. Atomic radius III. ...
- 14M.2.sl.TZ1.5d: Explain why the ionic radius of a chloride ion is greater than the atomic radius of a...
- 14M.2.sl.TZ1.6d: (i) Deduce the order of reactivity of these four metals, from the least to the most...
- 14M.1.sl.TZ2.8: Which pair of elements shows the greatest difference in electronegativity? A. Mg and...
- 14M.2.sl.TZ1.5b: (i) Predict any changes that may be observed in each...
- 14M.2.sl.TZ1.5e.ii: State the acid-base natures of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and...
- 14M.2.sl.TZ2.4a: (i) Describe the colour change that occurs when aqueous chlorine is added to aqueous...
- 14M.2.sl.TZ2.4b: The colour change in the reaction between aqueous chlorine and aqueous sodium iodide is very...
- 14N.1.hl.TZ0.6: Which statements are correct for the oxides of period 3 going from Na to Cl? I. The...
- 14N.1.hl.TZ0.7: The elements argon, potassium, and calcium are consecutive in the periodic table. Which gives...
- 14N.2.hl.TZ0.8c: (i) Magnesium reacts with oxygen to form an ionic compound, magnesium oxide. Describe how...
- 14N.1.sl.TZ0.7: Which properties decrease down both group 1 and group 7? I. Melting point II. First...
- 14N.1.sl.TZ0.8: Which period 3 oxide, when added to water, forms an acidic solution? A. ...
- 14N.2.sl.TZ0.6c: State an equation for the reaction of magnesium oxide with water.
- 13N.1.hl.TZ0.6: Which series is arranged in order of increasing radius? A. ...
- 13N.1.hl.TZ0.7: Which oxides form acidic solutions when added to water? A. ...
- 13N.2.hl.TZ0.6e.i: Compare the properties of the three oxides by completing the table below.
- 13N.2.hl.TZ0.7a: State the element that you would expect to have chemical properties most similar to those of...
- 13N.1.sl.TZ0.9: Which series is arranged in order of increasing radius? A. ...
- 13M.1.hl.TZ1.8: Each of the following oxides is added to separate equal volumes of distilled water. Which of...
- 13M.1.hl.TZ1.15: Which process is endothermic? A. ...
- 13M.2.hl.TZ1.7a.ii: Deduce, using equations where appropriate, if bromine reacts with sodium chloride solution...
- 13M.2.hl.TZ1.2a: Describe and explain the trend in atomic radius across period 3.
- 13M.2.hl.TZ1.2b: A student formulates the following hypothesis: “If phosphorus were to form a positive ion,...
- 13M.2.hl.TZ1.7a.i: Explain the trend in reactivity of the halogens.
- 13M.1.sl.TZ1.21: Which is a characteristic property of sodium oxide? A. It turns moist blue litmus paper...
- 13M.1.sl.TZ1.8: Which statement concerning electronegativity is correct? A. Electronegativity increases...
- 13M.1.sl.TZ1.9: Which statements are correct? I. Fluorine will react with potassium chloride solution to...
- 13M.2.sl.TZ1.2a: Describe and explain the trend in atomic radius across period 3.
- 13M.2.sl.TZ1.2b: A student formulates the following hypothesis: “If phosphorus were to form a positive ion,...
- 13M.1.hl.TZ2.7: Which statements are correct for the alkali metals Li to Cs? I. Melting point...
- 13M.1.sl.TZ2.9: Which statements are correct for the halogens F to I? I. Melting point increases II. ...
- 13M.2.sl.TZ2.6b.i: Define the term electronegativity.
- 13M.2.sl.TZ2.6b.ii: Using Table 7 of the Data Booklet, explain the trends in electronegativity values of the...
- 13M.2.sl.TZ2.6b.iii: State the balanced chemical equation for the reaction of potassium bromide, KBr(aq), with...
- 13M.2.sl.TZ2.6b.iv: Describe the colour change likely to be observed in this reaction.
- 12N.1.sl.TZ0.9: Which oxides are acidic? I. \({{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}\) II. ...
- 12N.1.sl.TZ0.8: Which combination is correct for the properties of the alkali metals from Li to Cs?
- 10N.1.hl.TZ0.7: The x-axis of the graph below represents the atomic number of the elements in period...
- 10N.2.hl.TZ0.4d: (i) State whether aqueous solutions of magnesium oxide and magnesium chloride are acidic,...
- 10N.1.sl.TZ0.8: Which properties of the alkali metals decrease going down group 1? A. First ionization...
- 10N.1.sl.TZ0.9: Which statements about the periodic table are correct? I. The elements Mg, Ca and Sr...
- 10N.1.sl.TZ0.10: The electronegativities of four different elements are given below (the letters are not their...
- 10N.2.sl.TZ0.4b: (i) Define the term electronegativity. (ii) Compare the relative polarities of the...
- 09N.1.hl.TZ0.8: Which species has the largest radius? A. Cl– B. K C. Na+ D. K+
- 09N.2.hl.TZ0.7a.v: The inter-ionic distance between the ions in NaF is very similar to that between the ions in...
- 09N.1.sl.TZ0.8: What happens when sodium is added to water? I. A gas is evolved II. The temperature...
- 10M.2.sl.TZ1.4e: State a balanced equation for the reaction of sodium with water. Include state symbols.
- 10M.2.sl.TZ1.4f: With reference to electronic arrangements, suggest why the reaction between rubidium and...
- 10M.2.sl.TZ1.4g: Describe and explain what you will see if chlorine gas is bubbled through a solution of (i) ...
- 10M.2.hl.TZ2.6c: (i) Outline two reasons why a sodium ion has a smaller radius than a sodium atom. (ii) ...
- 10M.1.sl.TZ2.8: Which property decreases down group 7 in the periodic table? A. Melting point B. ...
- 10M.1.sl.TZ2.9: Which oxides produce an acidic solution when added to water? I. ...
- 10M.2.sl.TZ2.5a: (i) Identify the property used to arrange the elements in the periodic table. (ii) ...
- 10M.2.sl.TZ2.3a: Explain the increase in the melting point from sodium to aluminium.
- 10M.2.sl.TZ2.3b: Explain why sulfur, \({{\text{S}}_{\text{8}}}\), has a higher melting point than phosphorus,...
- 10M.2.sl.TZ2.3c: Explain why silicon has the highest melting point and argon has the lowest melting point.
- 10M.2.sl.TZ2.5b: (i) Define the term first ionization energy of an atom. (ii) Explain the general...
- 09M.2.hl.TZ1.6b.iv: Explain why the quantitative value for the lattice enthalpy of calcium bromide is larger than...
- 09M.2.hl.TZ1.8b.ii: Explain how information from this graph provides evidence for the existence of main energy...
- 09M.2.hl.TZ1.8b.iv: Sketch and explain the shape of the graph obtained for the successive ionization energies of...
- 09M.1.sl.TZ1.10: Which is the best definition of electronegativity? A. Electronegativity is the energy...
- 09M.1.sl.TZ1.12: What happens when magnesium metal reacts with chlorine gas? A. Each magnesium atom loses...
- 09M.2.sl.TZ1.3c.ii: State the equation for the reaction of sodium oxide with water.
- 09M.2.sl.TZ1.5b.iii: A graph of atomic radius plotted against atomic number shows that the atomic radius decreases...
- 09M.2.sl.TZ1.5b.iv: Explain why a sulfide ion, \({{\text{S}}^{2 - }}\), is larger than a chloride ion,...
- 09M.2.sl.TZ1.3c.i: State the acid-base nature of sodium oxide.
- 09M.2.sl.TZ1.5b.i: Define the term first ionization energy and state what is meant by the term periodicity.
- 09M.2.sl.TZ1.5b.ii: State the electron arrangement of argon and explain why the noble gases, helium, neon and...
- 09M.2.sl.TZ1.5b.v: Explain why the melting points of the Group 1 metals \({\text{(Li}} \to {\text{Cs)}}\)...
- 09M.1.hl.TZ2.9: Which equation best represents the first ionization energy of magnesium? A. ...
- 09M.2.hl.TZ2.5d: Explain, using diagrams, why \({\text{N}}{{\text{O}}_{\text{2}}}\) is a polar molecule but...
- 09M.2.sl.TZ2.2a: Describe the acid-base character of the oxides of each of the period 3 elements, Na to Cl.
- 09M.1.sl.TZ2.8: Which statement describes the trends of electronegativity values in the periodic table? A. ...
- 09M.1.sl.TZ2.9: Which statement is correct for all elements in the same period? A. They have the same...
- 09M.2.sl.TZ2.7c: Explain, using diagrams, why CO and \({\text{N}}{{\text{O}}_{\text{2}}}\) are polar molecules...
- 11M.1.hl.TZ1.8: Which statement about the elements in group 7 is correct? A. ...
- 11M.2.hl.TZ1.6f.ii: Samples of sodium oxide and solid sulfur trioxide are added to separate beakers of water....
- 11M.1.sl.TZ1.8: Which property increases down group 1? A. First ionization energy B. Melting...
- 11M.1.sl.TZ1.7: Which property generally decreases across period 3? A. Atomic number B. ...
- 11M.1.sl.TZ1.12: Which combination of the characteristics of element X, a metal, and element Y, a non metal,...
- 11M.2.sl.TZ1.5a.ii: Explain why the first ionization energy of magnesium is higher than that of sodium.
- 11M.2.sl.TZ1.5b.i: calcium has a higher melting point than potassium.
- 11M.2.sl.TZ1.5a.i: Define the term first ionization energy.
- 11M.2.sl.TZ1.5d: Samples of sodium oxide and sulfur trioxide are added to separate beakers of water. Deduce...
- 11M.1.sl.TZ2.9: Which pair of elements has the greatest difference in electronegativity? A. Cs and...
- 11M.2.hl.TZ2.5b.iii: State the equations for the reactions of sodium oxide,...
- 11M.2.hl.TZ2.7e.ii: Explain why an aqueous solution of sodium chloride cannot be used to obtain sodium metal by...
- 11M.1.sl.TZ2.12: The number of electrons in the valence shell of elements A and B, are 6 and 7 respectively....
- 12M.1.sl.TZ2.9: Which series is correctly arranged in order of decreasing radius? A. ...
- 12M.2.sl.TZ2.3a: State the equation for the reaction between sodium and water.
- 12M.2.sl.TZ2.3b: State and explain one difference between the reactions of sodium and potassium with water.
- 11N.1.sl.TZ0.7: Which physical property of elements is represented by y on the graph below? A. First...
- 11N.2.sl.TZ0.1c.i: Suggest why it is necessary for sodium to be removed by this reaction.
- 11N.2.sl.TZ0.5a: Deduce the balanced chemical equation for the reaction between sodium and sulfur. State the...
- 11N.2.sl.TZ0.5b: Describe the acid-base character of the oxides of the period 3 elements, Na to Cl. For the...

