| Date | May 2015 | Marks available | 2 | Reference code | 15M.2.hl.TZ1.2 |
| Level | HL | Paper | 2 | Time zone | TZ1 |
| Command term | State | Question number | 2 | Adapted from | N/A |
Question
This question is about the compounds of some period 3 elements.
State the equations for the reactions of sodium oxide with water and phosphorus(V) oxide with water.
Explain why the melting point of phosphorus(V) oxide is lower than that of sodium oxide in terms of their bonding and structure.
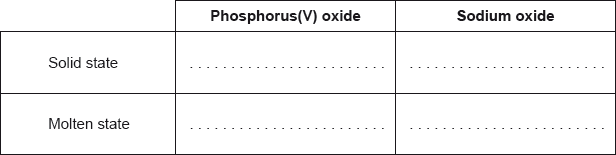
Predict whether phosphorus(V) oxide and sodium oxide conduct electricity in their solid and molten states. Complete the boxes with “yes” or “no”.
Predict and explain the pH of the following aqueous solutions, using equations to support your answer.
Ammonium chloride, \({\text{N}}{{\text{H}}_{\text{4}}}{\text{Cl(aq)}}\):
Sodium methanoate, \({\text{HCOONa(aq)}}\):
Markscheme
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O(s)}} + {{\text{H}}_{\text{2}}}{\text{O(l)}} \to {\text{2NaOH(aq);}}\)
Accept \(N{a_2}O(s) + {H_2}O(l) \to 2N{a^ + }(aq) + 2O{H^ - }(aq)\).
\({{\text{P}}_4}{{\text{O}}_{10}}{\text{(s)}} + {\text{6}}{{\text{H}}_2}{\text{O(l)}} \to {\text{4}}{{\text{H}}_3}{\text{P}}{{\text{O}}_4}{\text{(aq)}}\);
Accept \({P_2}{O_5}(s) + 3{H_2}O(l) \to 2{H_3}P{O_4}(aq)\).
Accept \({P_4}{O_{10}}(s) + 6{H_2}O(l) \to 4{H^ + }(aq) + 4{H_2}PO_4^ - (aq)\).
Ignore state symbols.
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) ionic and \({{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}\) covalent (within molecule);
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) in giant/3D/network/lattice structure with strong (ionic) bonds and \({{\text{P}}_{\text{4}}}{{\text{O}}_{{\text{10}}}}\) has a (simple) molecular structure/weak intermolecular forces (between molecules);
Award [1] for stating that bonds require more energy to break in \(N{a_2}O\) than in \({P_4}{O_{10}}\).
 ;
;
Award [2] for four correct.
Award [1] for two or three correct.
Ammonium chloride:
Accept any value in the range: \(3 < {\text{pH}} < 7\);
\({\text{NH}}_4^ + {\text{(aq)}} \rightleftharpoons {\text{N}}{{\text{H}}_3}{\text{(aq)}} + {{\text{H}}^ + }{\text{(aq)}}\);
Sodium methanoate:
\(7 < {\text{pH}} < 11\);
\({\text{HCO}}{{\text{O}}^ - }{\text{(aq)}} + {{\text{H}}_2}{\text{O(l)}} \rightleftharpoons {\text{HCOOH(aq)}} + {\text{O}}{{\text{H}}^ - }{\text{(aq)}}\);
Award [1 max] for both M1 and M3 combined if stated “pH < 7/acidic for ammonium chloride and pH > 7/alkaline/basic for sodium methanoate”.
Accept alternative suitable equations.
Award [1 max] for two correct explanations, such as “salt of weak acid and strong base” or “salt of weak base and strong acid”, without equations.
Penalize missing equilibrium sign once only.
Ignore state symbols.
Examiners report
In (a) sodium oxide was answered better than phosphorus(V) oxide (a direct reference to Assessment Statement 13.1.1) although there were many instances of NaO. In (b) there were the usual suggestions that covalent bonds are weaker than ionic bonds. Candidates find the distinction between inter- and intra-molecular bonding very difficult to grasp. Some didn’t realize that \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) is ionic. Answers about electrical conductivity usually gained one of the two marks available so there may have been an element of guesswork here. Many answers to (c) gained only one mark for knowing that one would be acidic and the other basic. There was very poor understanding of the equations needed and the explanation of the equilibria involved.
In (a) sodium oxide was answered better than phosphorus(V) oxide (a direct reference to Assessment Statement 13.1.1) although there were many instances of NaO. In (b) there were the usual suggestions that covalent bonds are weaker than ionic bonds. Candidates find the distinction between inter- and intra-molecular bonding very difficult to grasp. Some didn’t realize that \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) is ionic. Answers about electrical conductivity usually gained one of the two marks available so there may have been an element of guesswork here. Many answers to (c) gained only one mark for knowing that one would be acidic and the other basic. There was very poor understanding of the equations needed and the explanation of the equilibria involved.
In (a) sodium oxide was answered better than phosphorus(V) oxide (a direct reference to Assessment Statement 13.1.1) although there were many instances of NaO. In (b) there were the usual suggestions that covalent bonds are weaker than ionic bonds. Candidates find the distinction between inter- and intra-molecular bonding very difficult to grasp. Some didn’t realize that \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) is ionic. Answers about electrical conductivity usually gained one of the two marks available so there may have been an element of guesswork here. Many answers to (c) gained only one mark for knowing that one would be acidic and the other basic. There was very poor understanding of the equations needed and the explanation of the equilibria involved.
In (a) sodium oxide was answered better than phosphorus(V) oxide (a direct reference to Assessment Statement 13.1.1) although there were many instances of NaO. In (b) there were the usual suggestions that covalent bonds are weaker than ionic bonds. Candidates find the distinction between inter- and intra-molecular bonding very difficult to grasp. Some didn’t realize that \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) is ionic. Answers about electrical conductivity usually gained one of the two marks available so there may have been an element of guesswork here. Many answers to (c) gained only one mark for knowing that one would be acidic and the other basic. There was very poor understanding of the equations needed and the explanation of the equilibria involved.

