| Date | May 2014 | Marks available | 4 | Reference code | 14M.2.sl.TZ1.5 |
| Level | SL | Paper | 2 | Time zone | TZ1 |
| Command term | Describe, Predict, and State | Question number | 5 | Adapted from | N/A |
Question
Periodic trends enable chemists to predict the behaviour of related compounds.
Chlorine gas, \({\text{C}}{{\text{l}}_{\text{2}}}{\text{(g)}}\), is bubbled through separate solutions of aqueous bromine, \({\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}}\), and potassium bromide, \({\text{KBr(aq)}}\).
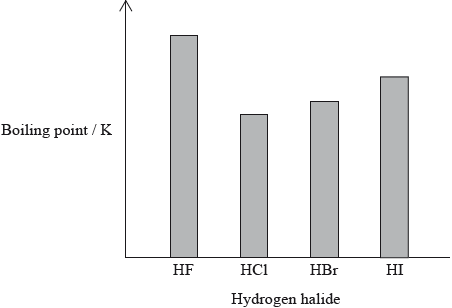
The hydrogen halides do not show perfect periodicity. A bar chart of boiling points shows that the boiling point of hydrogen fluoride, HF, is much higher than periodic trends would indicate.
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\) are two oxides of period 3 elements.
(i) State the equation for the reaction of sodium metal with water.
(ii) Describe two changes that could be observed during the reaction.
(iii) Predict the relative reaction rates of lithium, sodium and potassium with water.
(i) Predict any changes that may be observed in each case.
\({\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}}\):
\({\text{KBr(aq)}}\):
(ii) State the half-equations for the reactions that occur.
(i) Explain why the boiling point of HF is much higher than the boiling points of the other hydrogen halides.
(ii) Explain the trend in the boiling points of HCl, HBr and HI.
Explain why the ionic radius of a chloride ion is greater than the atomic radius of a chlorine atom.
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) does not conduct electricity in the solid state but it does when molten. Pure \({\text{S}}{{\text{O}}_{\text{3}}}\) does not conduct electricity in either the solid or liquid states.
Explain these facts.
State the acid-base natures of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\).
State equations for the reactions of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\) with water.
Markscheme
(i) \({\text{2Na(s)}} + {\text{2}}{{\text{H}}_2}{\text{O(l)}} \to {{\text{H}}_2}{\text{(g)}} + {\text{2NaOH(aq)}}/{\text{Na(s)}} + {{\text{H}}_2}{\text{O(l)}} \to \frac{1}{2}{{\text{H}}_2}{\text{(g)}} + {\text{NaOH(aq)}}\);
Ignore state symbols.
(ii) bubbles/gas produced / crackling / fizzing / OWTTE;
temperature (of water) increases;
sodium floats on water / melts into a ball / disappears / OWTTE;
sharp smell;
small yellow sparks;
(iii) K > Na > Li;
(i) \({\text{B}}{{\text{r}}_{\text{2}}}{\text{(aq)}}\): no change;
\({\text{KBr(aq)}}\): colour change / from colourless to red/yellow/orange/brown;
(ii) \({\text{2B}}{{\text{r}}^ - }{\text{(aq)}} \to {\text{B}}{{\text{r}}_2}{\text{(aq)}} + {\text{2}}{{\text{e}}^ - }\);
\({\text{C}}{{\text{l}}_2}{\text{(g)}} + {\text{2}}{{\text{e}}^ - } \to {\text{2C}}{{\text{l}}^ - }{\text{(aq)}}\);
Ignore state symbols.
Accept e instead of e–.
(i) HF has hydrogen bonds (between molecules);
(ii) strength of van der Waals’/London/dispersion forces increases;
as mass/size/number of electrons of halogen atom/molecule increases;
\({\text{C}}{{\text{l}}^ - }\) has an extra electron so extra repulsions push electrons farther apart / \({\text{C}}{{\text{l}}^ - }\) and Cl have same number of occupied electron shells and \({\text{C}}{{\text{l}}^ - }\) has one more electron than protons / Cl has 17 electrons and 17 protons and \({\text{C}}{{\text{l}}^ - }\) has 18 electrons and 17 protons so electrons are held less tightly / Cl 2, 8, 7 and \({\text{C}}{{\text{l}}^ - }\) 2, 8, 8 so electrons are held less tightly;
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) ionic and \({\text{S}}{{\text{O}}_{\text{3}}}\) covalent;
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) has ions which are free to move in the liquid state;
\({\text{S}}{{\text{O}}_{\text{3}}}\) has no free charged particles;
Accept “no free moving ions” / “no delocalized electrons”.
\({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) basic and \({\text{S}}{{\text{O}}_{\text{3}}}\) acidic;
\({\text{N}}{{\text{a}}_2}{\text{O(s)}} + {{\text{H}}_2}{\text{O(l)}} \to {\text{2NaOH(aq)}}\);
\({\text{S}}{{\text{O}}_3}{\text{(g)}} + {{\text{H}}_2}{\text{O(l)}} \to {{\text{H}}_2}{\text{S}}{{\text{O}}_4}{\text{(aq)}}\);
Ignore state symbols.
Examiners report
Question 5 was a popular choice in Section B. There were many candidates who stated a correct equation for the reaction of sodium with water, but many gave \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) instead of NaOH as a product in (a)(i). Candidates could frequently state one observation of this reaction in (a)(ii) and most candidates correctly predicted relative reaction rates for lithium, sodium and potassium with water in (a)(iii). Question (b)(i) required candidates to predict changes which may be observed when chlorine gas is bubbled through separate solutions of aqueous bromine and potassium bromide. Very few candidates correctly predicted both of the changes observed and there was some confusion between observations and statements of the expected reactions. In (b)(ii) many candidates gave the complete equation between chlorine and bromide ions and not the half-equations as requested. Part (c) referred to the periodicity of boiling points of hydrogen halides. Most candidates referred to the hydrogen bonding between HF molecules as the reason for the high boiling point of HF in (c)(i) although some said that the bond in H–F is a hydrogen bond and so hard to break, indicating a lack of understanding of what is happening on a molecular level when boiling occurs. Many had difficulties explaining the trend of the boiling points of the hydrogen halides, often referring to the elements themselves in (c)(ii). Only the better candidates referred to the strength of van der Waals’ forces increasing with increasing electrons or molecular mass. In part (d) very few candidates could explain why the ionic radius of a chloride ion is greater than the atomic radius of a chlorine atom, forgetting the extra repulsion between the electrons. Some answered in terms of the nuclear charge. Many simply stated that non-metal ions are larger than the non-metal atom, suggesting that the command terms are not well understood. Many candidates did not mention that \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) has an ionic bond and \({\text{S}}{{\text{O}}_{\text{3}}}\) a covalent one in (e)(i) and many candidates also had problems explaining why \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) conducts electricity when molten, referring to free moving electrons instead of ions. A surprising number of candidates seemed to think that as \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) melts, the sodium reverts to its metallic structure and hence is an electrical conductor. Several others referred to electrons being able to move through the ions which were no longer fixed in position. Several candidates could not state the acid-base nature of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\), although many could state their equations with water in (e)(iii). Many candidates correctly identified a source of \({\text{S}}{{\text{O}}_{\text{3}}}\) in (e)(iv) and those who did not often had a vague answer such as engine or factory. Most candidates correctly named acid rain as the environmental effect of sulfur trioxide pollution. Some respondents felt that this was beyond the scope of the syllabus but there is clear reference to these effects in 3.3.2 and 8.3.1.
Question 5 was a popular choice in Section B. There were many candidates who stated a correct equation for the reaction of sodium with water, but many gave \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) instead of NaOH as a product in (a)(i). Candidates could frequently state one observation of this reaction in (a)(ii) and most candidates correctly predicted relative reaction rates for lithium, sodium and potassium with water in (a)(iii). Question (b)(i) required candidates to predict changes which may be observed when chlorine gas is bubbled through separate solutions of aqueous bromine and potassium bromide. Very few candidates correctly predicted both of the changes observed and there was some confusion between observations and statements of the expected reactions. In (b)(ii) many candidates gave the complete equation between chlorine and bromide ions and not the half-equations as requested. Part (c) referred to the periodicity of boiling points of hydrogen halides. Most candidates referred to the hydrogen bonding between HF molecules as the reason for the high boiling point of HF in (c)(i) although some said that the bond in H–F is a hydrogen bond and so hard to break, indicating a lack of understanding of what is happening on a molecular level when boiling occurs. Many had difficulties explaining the trend of the boiling points of the hydrogen halides, often referring to the elements themselves in (c)(ii). Only the better candidates referred to the strength of van der Waals’ forces increasing with increasing electrons or molecular mass. In part (d) very few candidates could explain why the ionic radius of a chloride ion is greater than the atomic radius of a chlorine atom, forgetting the extra repulsion between the electrons. Some answered in terms of the nuclear charge. Many simply stated that non-metal ions are larger than the non-metal atom, suggesting that the command terms are not well understood. Many candidates did not mention that \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) has an ionic bond and \({\text{S}}{{\text{O}}_{\text{3}}}\) a covalent one in (e)(i) and many candidates also had problems explaining why \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) conducts electricity when molten, referring to free moving electrons instead of ions. A surprising number of candidates seemed to think that as \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) melts, the sodium reverts to its metallic structure and hence is an electrical conductor. Several others referred to electrons being able to move through the ions which were no longer fixed in position. Several candidates could not state the acid-base nature of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\), although many could state their equations with water in (e)(iii). Many candidates correctly identified a source of \({\text{S}}{{\text{O}}_{\text{3}}}\) in (e)(iv) and those who did not often had a vague answer such as engine or factory. Most candidates correctly named acid rain as the environmental effect of sulfur trioxide pollution. Some respondents felt that this was beyond the scope of the syllabus but there is clear reference to these effects in 3.3.2 and 8.3.1.
Question 5 was a popular choice in Section B. There were many candidates who stated a correct equation for the reaction of sodium with water, but many gave \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) instead of NaOH as a product in (a)(i). Candidates could frequently state one observation of this reaction in (a)(ii) and most candidates correctly predicted relative reaction rates for lithium, sodium and potassium with water in (a)(iii). Question (b)(i) required candidates to predict changes which may be observed when chlorine gas is bubbled through separate solutions of aqueous bromine and potassium bromide. Very few candidates correctly predicted both of the changes observed and there was some confusion between observations and statements of the expected reactions. In (b)(ii) many candidates gave the complete equation between chlorine and bromide ions and not the half-equations as requested. Part (c) referred to the periodicity of boiling points of hydrogen halides. Most candidates referred to the hydrogen bonding between HF molecules as the reason for the high boiling point of HF in (c)(i) although some said that the bond in H–F is a hydrogen bond and so hard to break, indicating a lack of understanding of what is happening on a molecular level when boiling occurs. Many had difficulties explaining the trend of the boiling points of the hydrogen halides, often referring to the elements themselves in (c)(ii). Only the better candidates referred to the strength of van der Waals’ forces increasing with increasing electrons or molecular mass. In part (d) very few candidates could explain why the ionic radius of a chloride ion is greater than the atomic radius of a chlorine atom, forgetting the extra repulsion between the electrons. Some answered in terms of the nuclear charge. Many simply stated that non-metal ions are larger than the non-metal atom, suggesting that the command terms are not well understood. Many candidates did not mention that \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) has an ionic bond and \({\text{S}}{{\text{O}}_{\text{3}}}\) a covalent one in (e)(i) and many candidates also had problems explaining why \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) conducts electricity when molten, referring to free moving electrons instead of ions. A surprising number of candidates seemed to think that as \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) melts, the sodium reverts to its metallic structure and hence is an electrical conductor. Several others referred to electrons being able to move through the ions which were no longer fixed in position. Several candidates could not state the acid-base nature of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\), although many could state their equations with water in (e)(iii). Many candidates correctly identified a source of \({\text{S}}{{\text{O}}_{\text{3}}}\) in (e)(iv) and those who did not often had a vague answer such as engine or factory. Most candidates correctly named acid rain as the environmental effect of sulfur trioxide pollution. Some respondents felt that this was beyond the scope of the syllabus but there is clear reference to these effects in 3.3.2 and 8.3.1.
Question 5 was a popular choice in Section B. There were many candidates who stated a correct equation for the reaction of sodium with water, but many gave \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) instead of NaOH as a product in (a)(i). Candidates could frequently state one observation of this reaction in (a)(ii) and most candidates correctly predicted relative reaction rates for lithium, sodium and potassium with water in (a)(iii). Question (b)(i) required candidates to predict changes which may be observed when chlorine gas is bubbled through separate solutions of aqueous bromine and potassium bromide. Very few candidates correctly predicted both of the changes observed and there was some confusion between observations and statements of the expected reactions. In (b)(ii) many candidates gave the complete equation between chlorine and bromide ions and not the half-equations as requested. Part (c) referred to the periodicity of boiling points of hydrogen halides. Most candidates referred to the hydrogen bonding between HF molecules as the reason for the high boiling point of HF in (c)(i) although some said that the bond in H–F is a hydrogen bond and so hard to break, indicating a lack of understanding of what is happening on a molecular level when boiling occurs. Many had difficulties explaining the trend of the boiling points of the hydrogen halides, often referring to the elements themselves in (c)(ii). Only the better candidates referred to the strength of van der Waals’ forces increasing with increasing electrons or molecular mass. In part (d) very few candidates could explain why the ionic radius of a chloride ion is greater than the atomic radius of a chlorine atom, forgetting the extra repulsion between the electrons. Some answered in terms of the nuclear charge. Many simply stated that non-metal ions are larger than the non-metal atom, suggesting that the command terms are not well understood. Many candidates did not mention that \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) has an ionic bond and \({\text{S}}{{\text{O}}_{\text{3}}}\) a covalent one in (e)(i) and many candidates also had problems explaining why \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) conducts electricity when molten, referring to free moving electrons instead of ions. A surprising number of candidates seemed to think that as \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) melts, the sodium reverts to its metallic structure and hence is an electrical conductor. Several others referred to electrons being able to move through the ions which were no longer fixed in position. Several candidates could not state the acid-base nature of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\), although many could state their equations with water in (e)(iii). Many candidates correctly identified a source of \({\text{S}}{{\text{O}}_{\text{3}}}\) in (e)(iv) and those who did not often had a vague answer such as engine or factory. Most candidates correctly named acid rain as the environmental effect of sulfur trioxide pollution. Some respondents felt that this was beyond the scope of the syllabus but there is clear reference to these effects in 3.3.2 and 8.3.1.
Question 5 was a popular choice in Section B. There were many candidates who stated a correct equation for the reaction of sodium with water, but many gave \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) instead of NaOH as a product in (a)(i). Candidates could frequently state one observation of this reaction in (a)(ii) and most candidates correctly predicted relative reaction rates for lithium, sodium and potassium with water in (a)(iii). Question (b)(i) required candidates to predict changes which may be observed when chlorine gas is bubbled through separate solutions of aqueous bromine and potassium bromide. Very few candidates correctly predicted both of the changes observed and there was some confusion between observations and statements of the expected reactions. In (b)(ii) many candidates gave the complete equation between chlorine and bromide ions and not the half-equations as requested. Part (c) referred to the periodicity of boiling points of hydrogen halides. Most candidates referred to the hydrogen bonding between HF molecules as the reason for the high boiling point of HF in (c)(i) although some said that the bond in H–F is a hydrogen bond and so hard to break, indicating a lack of understanding of what is happening on a molecular level when boiling occurs. Many had difficulties explaining the trend of the boiling points of the hydrogen halides, often referring to the elements themselves in (c)(ii). Only the better candidates referred to the strength of van der Waals’ forces increasing with increasing electrons or molecular mass. In part (d) very few candidates could explain why the ionic radius of a chloride ion is greater than the atomic radius of a chlorine atom, forgetting the extra repulsion between the electrons. Some answered in terms of the nuclear charge. Many simply stated that non-metal ions are larger than the non-metal atom, suggesting that the command terms are not well understood. Many candidates did not mention that \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) has an ionic bond and \({\text{S}}{{\text{O}}_{\text{3}}}\) a covalent one in (e)(i) and many candidates also had problems explaining why \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) conducts electricity when molten, referring to free moving electrons instead of ions. A surprising number of candidates seemed to think that as \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) melts, the sodium reverts to its metallic structure and hence is an electrical conductor. Several others referred to electrons being able to move through the ions which were no longer fixed in position. Several candidates could not state the acid-base nature of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\), although many could state their equations with water in (e)(iii). Many candidates correctly identified a source of \({\text{S}}{{\text{O}}_{\text{3}}}\) in (e)(iv) and those who did not often had a vague answer such as engine or factory. Most candidates correctly named acid rain as the environmental effect of sulfur trioxide pollution. Some respondents felt that this was beyond the scope of the syllabus but there is clear reference to these effects in 3.3.2 and 8.3.1.
Question 5 was a popular choice in Section B. There were many candidates who stated a correct equation for the reaction of sodium with water, but many gave \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) instead of NaOH as a product in (a)(i). Candidates could frequently state one observation of this reaction in (a)(ii) and most candidates correctly predicted relative reaction rates for lithium, sodium and potassium with water in (a)(iii). Question (b)(i) required candidates to predict changes which may be observed when chlorine gas is bubbled through separate solutions of aqueous bromine and potassium bromide. Very few candidates correctly predicted both of the changes observed and there was some confusion between observations and statements of the expected reactions. In (b)(ii) many candidates gave the complete equation between chlorine and bromide ions and not the half-equations as requested. Part (c) referred to the periodicity of boiling points of hydrogen halides. Most candidates referred to the hydrogen bonding between HF molecules as the reason for the high boiling point of HF in (c)(i) although some said that the bond in H–F is a hydrogen bond and so hard to break, indicating a lack of understanding of what is happening on a molecular level when boiling occurs. Many had difficulties explaining the trend of the boiling points of the hydrogen halides, often referring to the elements themselves in (c)(ii). Only the better candidates referred to the strength of van der Waals’ forces increasing with increasing electrons or molecular mass. In part (d) very few candidates could explain why the ionic radius of a chloride ion is greater than the atomic radius of a chlorine atom, forgetting the extra repulsion between the electrons. Some answered in terms of the nuclear charge. Many simply stated that non-metal ions are larger than the non-metal atom, suggesting that the command terms are not well understood. Many candidates did not mention that \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) has an ionic bond and \({\text{S}}{{\text{O}}_{\text{3}}}\) a covalent one in (e)(i) and many candidates also had problems explaining why \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) conducts electricity when molten, referring to free moving electrons instead of ions. A surprising number of candidates seemed to think that as \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) melts, the sodium reverts to its metallic structure and hence is an electrical conductor. Several others referred to electrons being able to move through the ions which were no longer fixed in position. Several candidates could not state the acid-base nature of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\), although many could state their equations with water in (e)(iii). Many candidates correctly identified a source of \({\text{S}}{{\text{O}}_{\text{3}}}\) in (e)(iv) and those who did not often had a vague answer such as engine or factory. Most candidates correctly named acid rain as the environmental effect of sulfur trioxide pollution. Some respondents felt that this was beyond the scope of the syllabus but there is clear reference to these effects in 3.3.2 and 8.3.1.
Question 5 was a popular choice in Section B. There were many candidates who stated a correct equation for the reaction of sodium with water, but many gave \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) instead of NaOH as a product in (a)(i). Candidates could frequently state one observation of this reaction in (a)(ii) and most candidates correctly predicted relative reaction rates for lithium, sodium and potassium with water in (a)(iii). Question (b)(i) required candidates to predict changes which may be observed when chlorine gas is bubbled through separate solutions of aqueous bromine and potassium bromide. Very few candidates correctly predicted both of the changes observed and there was some confusion between observations and statements of the expected reactions. In (b)(ii) many candidates gave the complete equation between chlorine and bromide ions and not the half-equations as requested. Part (c) referred to the periodicity of boiling points of hydrogen halides. Most candidates referred to the hydrogen bonding between HF molecules as the reason for the high boiling point of HF in (c)(i) although some said that the bond in H–F is a hydrogen bond and so hard to break, indicating a lack of understanding of what is happening on a molecular level when boiling occurs. Many had difficulties explaining the trend of the boiling points of the hydrogen halides, often referring to the elements themselves in (c)(ii). Only the better candidates referred to the strength of van der Waals’ forces increasing with increasing electrons or molecular mass. In part (d) very few candidates could explain why the ionic radius of a chloride ion is greater than the atomic radius of a chlorine atom, forgetting the extra repulsion between the electrons. Some answered in terms of the nuclear charge. Many simply stated that non-metal ions are larger than the non-metal atom, suggesting that the command terms are not well understood. Many candidates did not mention that \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) has an ionic bond and \({\text{S}}{{\text{O}}_{\text{3}}}\) a covalent one in (e)(i) and many candidates also had problems explaining why \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) conducts electricity when molten, referring to free moving electrons instead of ions. A surprising number of candidates seemed to think that as \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) melts, the sodium reverts to its metallic structure and hence is an electrical conductor. Several others referred to electrons being able to move through the ions which were no longer fixed in position. Several candidates could not state the acid-base nature of \({\text{N}}{{\text{a}}_{\text{2}}}{\text{O}}\) and \({\text{S}}{{\text{O}}_{\text{3}}}\), although many could state their equations with water in (e)(iii). Many candidates correctly identified a source of \({\text{S}}{{\text{O}}_{\text{3}}}\) in (e)(iv) and those who did not often had a vague answer such as engine or factory. Most candidates correctly named acid rain as the environmental effect of sulfur trioxide pollution. Some respondents felt that this was beyond the scope of the syllabus but there is clear reference to these effects in 3.3.2 and 8.3.1.

